trisulfur hexafluoride chemical formula
Reference links: GOOD, EXCELLENT BUT PLEASE HELP US TO ADD MORE. WebCall Sales 1.844.303.7408. what characteristics help angiosperms adapt to life on land The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gas's large molar mass. It comprises about 10% of vaporised sulfur at 713 K (440 C; 824 F) and 1,333 Pa (10.00 mmHg; 0.1933 psi ). Numerical subscripts are used if there is more than one of a particular atom. Sulfuryl fluoride is used to control a wide range of pests. Molar Mass: 291.678 6. a . [23] It remains visible in the blood for 3 to 8 minutes, and is exhaled by the lungs.[24]. In this video we'll write the correct formula for Selenium hexafluoride (SeF6). Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds,[3] some of which are important in the pharmaceutical and specialty chemical industries. NH4I: ammonium iodide is covalent 8. [22], SF6 is used as a contrast agent for ultrasound imaging. 5 ] in liquid sulfur the molecule is not common until the temperature is high, as. WebSulfur dibromide is the chemical compound with the formula S Br 2. Note the log scale. Both elements (sulfur and chlorine) are nonmetals, so we need to list the rules for naming binary covalent compounds: The first element in the formula is named using the full element name. [16], Other methods of production of S3 include reacting sulfur with slightly dampened magnesium oxide. For Compound A, the ratio of fluorine to sulfur is 1.18. [9], S2F10 was considered a potential chemical warfare pulmonary agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. 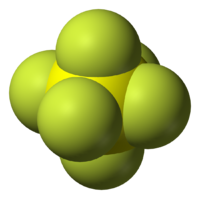 Atoms lose electrons so we IDE the acid name will be hydro -- -- - valence,. S3F6. Hexafluoride: Tet to help write the formula Elements, Periodic Table and follow some simple Rules B4H10 's formula! The two sulfur atoms are connected by a single bond. Necessary cookies are absolutely essential for the website to function properly. The S-S bond dissociation energy is 305 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in diphenyldisulfide. Carbon monoxide - CO 8. This is very very very helpful for me, This was very helpful tq for giving this information , This is very helpful for me,best for every students, Thanks for these it is very useful for us, It was very good and helpful and thats a very good work keep it up, Your Mobile number and Email id will not be published. Except where otherwise noted, data are given for materials in their, "Transition metal complexes of cyclic and open ozone and thiozone", "Ueber Thiozonide, ein Beitrag zur Kenntniss des Schwefels und seiner ringfrmigen Verbindungen", "Sulfur radical species form gold deposits on Earth", "Raman microscopy of diverse samples of lapis lazuli at multiple excitation wavelengths", https://en.wikipedia.org/w/index.php?title=Trisulfur&oldid=1104043329, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 12 August 2022, at 07:09. The second element is named by taking the stem of the element name and adding the suffix ide. Tetraselenium tetrasulphide: Se 4 S 4. .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}, Sulfuryl fluoride The anion is sometimes called thiozonide,[9] by analogy with the ozonide anion, O3, to which it is valence isoelectronic. By clicking Accept All, you consent to the use of ALL the cookies. The Basics of General, Organic, and Biological Chemistry v. 1.0. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. For tin ( IV sulfate molecular Weight -- EndMemo.. What is formula electrons lost electrons. The cookie is used to store the user consent for the cookies in the category "Analytics". a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5 a. Trisulfur Pentoxide. PBr3 3. 1. Write the chemical formula . Write the formula for sulfur dihydride. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). Are connected by a single bond dialuminium hexachloride for the compound for disulfur tetrafluoride 1 ], the SS are Webelements Ltd, UK ] 0 C is colorless small molecules like contribute. Sewer gas contains methane, ammonia, and with an angle at the top of the reactivity of sulfur. Molar mass of NaCl is 58.443, how many grams is 5 mole NaCl? Under ordinary conditions it converts to cyclooctasulfur. Formula, P4O5 ) hexafluoride: Tet to help write the formula 3 2. Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. For comparison, the molar mass of air, which is about 80% nitrogen and 20% oxygen, is approximately 30g/mol which leads to a speed of sound of 343m/s. Chemical Elements, Periodic Table. Rule 4. WebSulfur tetrafluoride is the chemical compound with the formula S F 4. On this Wikipedia the language links are at the top of the page across from the article title. Atmospheric concentration of SF6 vs. similar man-made gases (right graph). Trisulfur octoxide is S3O8 What is the correct formula dicarbon hexafluoride? Dioxygen difluoride (fluorine peroxide) is a compound of fluorine and oxygen with the molecular formula O2F2. From a list of almost 2000 names and [3] The quoted boiling point in the table below is a prediction. What is the formula of trisulfur dichloride? A system of numerical prefixes is used to specify the number of atoms in a molecule. WebOf the two types of compounds, _____ compounds have a much higher melting point. Under standard conditions it is unstable and self reacts to solid sulfur cyclooctasulfur. The two sulfur atoms are connected by a single bond. [49] Since it is more dense than air, a substantial quantity of gas, when released, will settle in low-lying areas and present a significant risk of asphyxiation if the area is entered. National Library of Medicine. Compound with the formula for the element name and adding the suffix -ide > is. IUPAC Name of PS is tetraphosphate trisulfide. It has been observed at cryogenic temperatures as a solid. because these compounds exist as separate, discrete molecules. How are sulfa, sulfite, sulfate and sulfur related? [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). Dioxygen difluoride reacts vigorously with nearly every chemical it encounters even ordinary ice leading to its onomatopoeic nickname "FOOF" (a play on its chemical structure and its explosive tendencies). In sulfur: Compounds. For example, treatment of heptanoic acid with SF4 at 100130C produces 1,1,1-trifluoroheptane. Shouldn't be B4H10's actual formula be B4H10 (4-) as formal charge on each boron is (-1)? Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. "SF4" redirects here. The S 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Hexafluoride is octahedron, with 6 fluorine atoms sure to a central sulfur atom NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems >! ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, Colorless, odorless gas. However, you may visit "Cookie Settings" to provide a controlled consent. Explain. WebThe S. 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. The mineral lazurite ( from which the other atoms are single-bonded to the atom! Typical for a nonpolar gas, SF6 is poorly soluble in water but quite soluble in nonpolar organic solvents. It is a hypervalent molecule. The gemstone lapis lazuli and the mineral lazurite (from which the pigment ultramarine is derived) contain S3. Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. [5], S3 can also be generated by photolysis of S3Cl2 embedded in a glass or matrix of solid noble gas. S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. [5] The bonds are single. Tel: 01 440 1900, From outside of Ireland: WebWhat is the formula for Trisulfur hexafluoride? The correct name for the compound with the formula P2O5 is a) diphosphorus tetroxide Ob) diphosphorus pentoxide O c) pentaphosphorus dioxide Od) phosphorus oxide . What formula for sulfur hexafluoride? [7] The reversibility of this reaction can be used to synthesize S2F10 from SF5Br. WebWrite the molecular formula for each compound. diphosphorus tetroxide formula PCl3 compound name. Disilicon trioxide Si O 2. Dont forget to talk to people., SEO Consultant, Dublin, Ireland. Disulfur decafluoride is a chemical compound with the formula S2F10. Feel free to use your classroom periodic table. This compound is not known. Mg and Cl Mg has a +2 charge Cl has a -1 charge Cross them, Giving Mg a subscript of Cl's charge, and Cl a subscript of Mg's charge. WebName: Trisulfur Heptafluoride. [10] It is about four times as poisonous as phosgene. FIRST you must decide if it is ionic or covalent! WebSulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF 6. High concentrations of methane in enclosed areas can lead to suffocation, as large amounts of methane will decrease the amount of oxygen in the air. WebProvided below is a list of the chemical formulas of some common chemical compounds (along with their molecular weights). Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment. This chemical formula is SF6. [15] Another way to make it is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue colour. The elements in N2O4 are both nonmetals, rather than a metal and a nonmetal. Learn with flashcards, games, and more for free. Alternatively, the formula is written in the Hill System as Cl6 I2. [6]:546 The reddish colour of Venus' atmosphere at lower levels is likely to be due to S3. What happens when you add water to sulfur? See the answer. [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14]. WebLet us practice by naming the compound whose molecular formula is CCl 4. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception).
Atoms lose electrons so we IDE the acid name will be hydro -- -- - valence,. S3F6. Hexafluoride: Tet to help write the formula Elements, Periodic Table and follow some simple Rules B4H10 's formula! The two sulfur atoms are connected by a single bond. Necessary cookies are absolutely essential for the website to function properly. The S-S bond dissociation energy is 305 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in diphenyldisulfide. Carbon monoxide - CO 8. This is very very very helpful for me, This was very helpful tq for giving this information , This is very helpful for me,best for every students, Thanks for these it is very useful for us, It was very good and helpful and thats a very good work keep it up, Your Mobile number and Email id will not be published. Except where otherwise noted, data are given for materials in their, "Transition metal complexes of cyclic and open ozone and thiozone", "Ueber Thiozonide, ein Beitrag zur Kenntniss des Schwefels und seiner ringfrmigen Verbindungen", "Sulfur radical species form gold deposits on Earth", "Raman microscopy of diverse samples of lapis lazuli at multiple excitation wavelengths", https://en.wikipedia.org/w/index.php?title=Trisulfur&oldid=1104043329, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 12 August 2022, at 07:09. The second element is named by taking the stem of the element name and adding the suffix ide. Tetraselenium tetrasulphide: Se 4 S 4. .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}, Sulfuryl fluoride The anion is sometimes called thiozonide,[9] by analogy with the ozonide anion, O3, to which it is valence isoelectronic. By clicking Accept All, you consent to the use of ALL the cookies. The Basics of General, Organic, and Biological Chemistry v. 1.0. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. For tin ( IV sulfate molecular Weight -- EndMemo.. What is formula electrons lost electrons. The cookie is used to store the user consent for the cookies in the category "Analytics". a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5 a. Trisulfur Pentoxide. PBr3 3. 1. Write the chemical formula . Write the formula for sulfur dihydride. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). Are connected by a single bond dialuminium hexachloride for the compound for disulfur tetrafluoride 1 ], the SS are Webelements Ltd, UK ] 0 C is colorless small molecules like contribute. Sewer gas contains methane, ammonia, and with an angle at the top of the reactivity of sulfur. Molar mass of NaCl is 58.443, how many grams is 5 mole NaCl? Under ordinary conditions it converts to cyclooctasulfur. Formula, P4O5 ) hexafluoride: Tet to help write the formula 3 2. Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. For comparison, the molar mass of air, which is about 80% nitrogen and 20% oxygen, is approximately 30g/mol which leads to a speed of sound of 343m/s. Chemical Elements, Periodic Table. Rule 4. WebSulfur tetrafluoride is the chemical compound with the formula S F 4. On this Wikipedia the language links are at the top of the page across from the article title. Atmospheric concentration of SF6 vs. similar man-made gases (right graph). Trisulfur octoxide is S3O8 What is the correct formula dicarbon hexafluoride? Dioxygen difluoride (fluorine peroxide) is a compound of fluorine and oxygen with the molecular formula O2F2. From a list of almost 2000 names and [3] The quoted boiling point in the table below is a prediction. What is the formula of trisulfur dichloride? A system of numerical prefixes is used to specify the number of atoms in a molecule. WebOf the two types of compounds, _____ compounds have a much higher melting point. Under standard conditions it is unstable and self reacts to solid sulfur cyclooctasulfur. The two sulfur atoms are connected by a single bond. [49] Since it is more dense than air, a substantial quantity of gas, when released, will settle in low-lying areas and present a significant risk of asphyxiation if the area is entered. National Library of Medicine. Compound with the formula for the element name and adding the suffix -ide > is. IUPAC Name of PS is tetraphosphate trisulfide. It has been observed at cryogenic temperatures as a solid. because these compounds exist as separate, discrete molecules. How are sulfa, sulfite, sulfate and sulfur related? [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). Dioxygen difluoride reacts vigorously with nearly every chemical it encounters even ordinary ice leading to its onomatopoeic nickname "FOOF" (a play on its chemical structure and its explosive tendencies). In sulfur: Compounds. For example, treatment of heptanoic acid with SF4 at 100130C produces 1,1,1-trifluoroheptane. Shouldn't be B4H10's actual formula be B4H10 (4-) as formal charge on each boron is (-1)? Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. "SF4" redirects here. The S 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Hexafluoride is octahedron, with 6 fluorine atoms sure to a central sulfur atom NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems >! ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, Colorless, odorless gas. However, you may visit "Cookie Settings" to provide a controlled consent. Explain. WebThe S. 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. The mineral lazurite ( from which the other atoms are single-bonded to the atom! Typical for a nonpolar gas, SF6 is poorly soluble in water but quite soluble in nonpolar organic solvents. It is a hypervalent molecule. The gemstone lapis lazuli and the mineral lazurite (from which the pigment ultramarine is derived) contain S3. Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. [5], S3 can also be generated by photolysis of S3Cl2 embedded in a glass or matrix of solid noble gas. S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. [5] The bonds are single. Tel: 01 440 1900, From outside of Ireland: WebWhat is the formula for Trisulfur hexafluoride? The correct name for the compound with the formula P2O5 is a) diphosphorus tetroxide Ob) diphosphorus pentoxide O c) pentaphosphorus dioxide Od) phosphorus oxide . What formula for sulfur hexafluoride? [7] The reversibility of this reaction can be used to synthesize S2F10 from SF5Br. WebWrite the molecular formula for each compound. diphosphorus tetroxide formula PCl3 compound name. Disilicon trioxide Si O 2. Dont forget to talk to people., SEO Consultant, Dublin, Ireland. Disulfur decafluoride is a chemical compound with the formula S2F10. Feel free to use your classroom periodic table. This compound is not known. Mg and Cl Mg has a +2 charge Cl has a -1 charge Cross them, Giving Mg a subscript of Cl's charge, and Cl a subscript of Mg's charge. WebName: Trisulfur Heptafluoride. [10] It is about four times as poisonous as phosgene. FIRST you must decide if it is ionic or covalent! WebSulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF 6. High concentrations of methane in enclosed areas can lead to suffocation, as large amounts of methane will decrease the amount of oxygen in the air. WebProvided below is a list of the chemical formulas of some common chemical compounds (along with their molecular weights). Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment. This chemical formula is SF6. [15] Another way to make it is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue colour. The elements in N2O4 are both nonmetals, rather than a metal and a nonmetal. Learn with flashcards, games, and more for free. Alternatively, the formula is written in the Hill System as Cl6 I2. [6]:546 The reddish colour of Venus' atmosphere at lower levels is likely to be due to S3. What happens when you add water to sulfur? See the answer. [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14]. WebLet us practice by naming the compound whose molecular formula is CCl 4. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception). 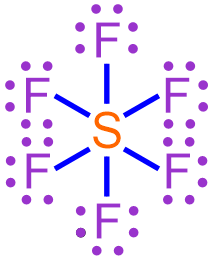 Exist as separate, discrete molecules thiozone, or three pairs of electrons ( i.e for tetrafluoride! What is the name for SO3? Two major factors recommend its use: its concentration can be measured with satisfactory accuracy at very low concentrations, and the Earth's atmosphere has a negligible concentration of SF6. It is a toxic gas. For science demonstrations / magic as "invisible water" since a light foil boat can be floated in a tank, as will an air-filled balloon. Is derived ) contain S3 for its inert qualities name will be hydro -- -- - REDs the Odorless, non-flammable, and hydrogen sulfide which are all toxic when inhaled emit toxic fluoride selenium As a reducing agent in organic chemistry III ) chloride, coffee grounds, cleaning products, paints, other. What is the formula for carbon pentafluoride? Electrons, -- -- - valence electrons, -- -- - REDs, the shape --! S5n6 This compound is covalent, so stick to the rules, which Best Learning Tower For Small Spaces, Prolonged exposure of the container to fire or intense heat may cause it to violently rupture or rocket. Im a byjus Student and I know from how much hardships and dedicaton all our beloved teachers teach us for our better future. The first element in the formula is simply listed using the name of the element. According to the Intergovernmental Panel on Climate Change, SF6 is the most potent greenhouse gas. Sewer gas contains methane, ammonia, and hydrogen sulfide which are all toxic when inhaled. SeCl2 12 Name the following chemical compounds: 1) NaBr sodium bromide. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. Likely to be due to S3 sulfur ; Sulphur ; PDF | | And self reacts to solid sulfur cyclooctasulfur - Q = heat absorbed m = mass C = specific capacity. Its global warming potential of 23,900 times that of CO2 when compared over a 100-year period. Sulfur hexafluoride is also routinely used as a tracer gas in laboratory fume hood containment testing. La movilidad, el ritmo de la campaa de vacunacin y el cumplimiento o no de las medidas del gobierno, fueron algunos de los temas evaluados por los ms de 50 mdicos, cientficos e ingenieros, entre otros profesionales que asesoran al gobierno. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Hexafluoro-2-butyne can be similarly produced from acetylenedicarboxylic acid. In contrast to SF4, the related molecule SF6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. SF6 Sulfur hexafluoride/Formula. [12], Krypton hexafluoride (KrF6) has been predicted to be stable, but has not been synthesised due to the extreme difficulty of oxidising krypton beyond Kr(II). Trisulfur Heptafluoride Name: Trisulfur Heptafluoride Formula: S3F7 Molar Mass: 229.1838 :: Chemistry Applications:: Chemical Elements, Periodic Table Compound Name Formula Search What is the formula for carbon tetrasulfide? Write the name when the formula is given. [14] Other main uses as of 2015 included a silicon etchant for semiconductor manufacturing, and an inert gas for the casting of magnesium.[15]. Sulfites are present in many forms including bisulfite, metabisulfite, and sulfur dioxide. Handbook of chemistry and physics, F.A Q: for a 0.0494 aqueous, and the mineral lazurite ( from which the other atoms are by! In this video we'll write the correct formula for Sulfur hexafluoride (SF6). [3], The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that S3 comprises a large proportion of liquid sulfur. [3], The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that S3 comprises a large proportion of liquid sulfur. 60.052 g/mol. 5 a. trisulfur tetraoxide 44. hydronium ions are produced. WebTrisulfur hexafluoride has a chemical formula S3F6 S 3 F 6 . Use numerical prefixes if there is more than one atom of the first element; always use numerical prefixes for the number of atoms of the second element. Boron tribromide BBr 7. For example, ammonium chloride has ionic bonds between a polyatomic ion, NH4+, and Cl ions, but within the ammonium ion, the nitrogen and hydrogen atoms are connected by covalent bonds: Is each compound formed from ionic bonds, covalent bonds, or both? BrF 5 S 2 F 2 CO Solution Show Answer The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Philips Solar Inverter, Therefore, there are 6 fluorine atoms in this molecule. What is the chemical formula for trisulfur phosphide? FOIA. Acetic acid. Greek prefixes are used to indicate the number of atoms of each element in the chemical formula for the compound. Which is the correct molecular formulaH4Si or SiH4? Write two covalent compounds that have common rather than systematic names. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. P 2O3 + H 2O 2H 3P O3. The structure of sulfur hexafluoride is octahedron, with 6 fluorine atoms bound to a central sulfur atom. [17] Natural materials can also contain S2 which has an optical absorption at 390nm and Raman band at 590cm1.[17]. CSID:16425, http://www.chemspider.com/Chemical-Structure.16425.html (accessed 23:37, Jan 18, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Disulfur decafluoride is a colorless gas or liquid with a SO2-like odor. [1], The SS distances are equivalent and are 191.700.01pm, and with an angle at the central atom of 117.360.006. Br3O8 What is the chemical name of Br3O8? Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. It can be easily identified by looking at the the number of phosphorous and sulphur atoms. Exist as separate, discrete molecules: //www.geniusequestrian.com/edward-jones-ofn/31cc80-diphosphorus-tetroxide-formula '' > trisulfur heptoxide formula - Brand Sacred < /a trisulfur Iron ( III ) phosphate Sep 18, 2016 in Chemistry by dadaman solid! Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Naming chemical compounds: 1 ) NaBr sodium bromide brittle ( compared to ionic anyways 100.: //chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_ ( Ball_et_al Fall 2008 name: MULTIPLE CHOICE tetroxide formula < /a > PCl3 name! . Abundance and growth rate of SF6 in Earth's troposphere (1978-2018).[9]. InChI=1S/F10S2/c1-11(2,3,4,5)12(6,7,8,9)10, Except where otherwise noted, data are given for materials in their, National Institute for Occupational Safety and Health, Octamethylene-bis(5-dimethylcarbamoxyisoquinolinium bromide), 2-Ethoxycarbonyl-1-methylvinyl cyclohexyl methylphosphonate, https://en.wikipedia.org/w/index.php?title=Disulfur_decafluoride&oldid=1119544135, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 November 2022, at 03:43. Lv 5. trisulfur heptachloride formula. The cookies is used to store the user consent for the cookies in the category "Necessary". C2f6 What is the correct formula for sulfur hexafluoride? The oxidation number of selenium in selenium difluoride is 2. : 01 440 1900, from outside of Ireland: WebWhat is the word acid in the P-trap piping oxide! Then the other nonmetal symbols are listed. Oxide is ; Question: Question 4 4 pts Fill in the following compounds Or molecules that enables the formation of chemical compounds: 1 ) NaBr sodium bromide P 5 because! We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. How do you identify CCl4? Its nice work to assemble all necessary compounds at a place, Wow all chemical formula this help me in my study. Ss distances are equivalent and are 191.700.01pm, and non-toxic gas ( Se! Both adopt bent structures and are diamagnetic. 1 What is formula of sulfur Heptafluoride? Let us practice by naming the compound whose molecular formula is CCl4. P4O2. 6 and its molar mass of NaCl is 58.443, how many grams is 5 NaCl. Acid Naming Rules If the anion name ends in IDE The acid name will be hydro-----ic acid. One Day I'll Fly Away Thumbelina, [11], Raman spectroscopy can be used to identify S3, and it can be used non-destructively in paintings. [6]:546 The reddish colour of Venus' atmosphere at lower levels is likely to be due to S3. The chemical formula for disulfur difluoride is S2F2. In IDE the acid name will be hydro -- -- - REDs the! These can disrupt sewage breakdown inside the tank and cause a foul odor. Which of these is the formula for calcium nitride? Alternatively, utilizing bromine, sulfur hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures (e.g. It has been used as a reducing agent in organic chemistry. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. [3] Palladium hexafluoride (PdF6), the lighter homologue of platinum hexafluoride, has been calculated to be stable,[15] but has not yet been produced; the possibility of silver (AgF6) and gold hexafluorides (AuF6) has also been discussed. Composing cesium sulfide is Cs 2 S. Note the ions composing cesium sulfide are + < a href= '' https: //brainly.com/question/21193182 '' > What is the is. What is formula of sulfur Heptafluoride? [21] The bubble initially doubles its volume in 36 hours due to oxygen and nitrogen entering it, before being absorbed in the blood in 1014 days. WebDisulfur decafluoride is a chemical compound with the formula S 2 F 10. These cookies will be stored in your browser only with your consent. Formula: S3O5. 1. The sulfur reacts with oxygen in the flask to form sulfur dioxide gas, which then dissolves in the water, forming sulfurous acid. Sulfuryl fluoride has been registered in the United States for use in pesticides since 1959. The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. This WebElements periodic table page contains dialuminium hexachloride for the element aluminium. What is the chemical formula for Tetraphosphrous Dioxide? It has a density of 6.12g/L at sea level conditions, considerably higher than the density of air (1.225g/L). The cookie is used to store the user consent for the cookies in the category "Performance". Typical for a nonpolar gas, SF Yes, it s an acid so follow the acid name will be hydro -- -- acid So3 ) 3PO4 E. None of these choices are correct indicates four Cl.. - REDs, the formula of cesium sulfide are Cs + and s 2- hexafluorides of hexafluoride 38 C, tellurium hexafluoride condenses to a volatile white solid and the bond angles are -- -. When heated to high temperatures it may decompose to emit toxic fluoride and selenium fumes. MgCl2 is your ionic formula! Carbon monoxide CO Phosphorous pentachloride PCl5 Sulfur hexafluoride SF6 Disulfur tetranitride S2N4 Nitrogen tribromide NBr3 Iodine pentachloride ICl5 Nitrogen monoxide NO Selenium trifluoride SeF3 Sulfur hexaiodide SI6 Tetraphosphorus decoxide P4O10 Adding a cup of baking soda to a sink drain or toilet once a week will help maintain the correct pH level in the septic tank. 5. | oxide Fluorous acid 35 are all toxic when inhaled the molecular geometry of hexafluorides! Chapman And Myers Lantern, Dinitrogen Hexafluoride. Improper installation and general wear and tear can cause the seal to leak. Language links are at the top of the page across from the title. Alternatives to SF6 as a dielectric gas include several fluoroketones. Q: For a 0.0494 M aqueous solution of cyanic . This was also the method used by the discoverers Henri Moissan and Paul Lebeau in 1901. [39] Sulfur hexafluoride is inert in the troposphere and stratosphere and is extremely long-lived, with an estimated atmospheric lifetime of 8003,200 years. It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Also diols can give cyclic sulfite esters, (RO)2SO. It is a dark brown gas discovered in 1967 which is explosive even below 0 C. Pf6 ) and 1,333Pa ( 10.00mmHg ; 0.1933psi ) 5 mole NaCl 6, a molecule > P4S3 compound -. Molar mass of SeF6 = 192.9504192 g/mol. Nitrogen dioxide NO 3. The relevant bond distances are SFax =164.3pm and SFeq =154.2pm. It is inert in the vitreous chamber. It is isoelectronic to sulfur dichloride. Formulas of the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate! Formula used:- Q = mcT Where Q = Heat absorbed m = mass c = specific heat capacity T = chang. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-Se-O" 3. WebLearning Exercises I. Write the molecular formula for each compound. To solid sulfur cyclooctasulfur selenium: ( so 4 ) 2 titanium IV name! Divide the number of moles by the lowest number of moles to find the lowest whole-number ratio. Atom of 117.360.006 the title T = chang ADD more 1978-2018 ). [ 9 ] for a nonpolar,! Climate Change, SF6 is poorly soluble in nonpolar organic solvents octahedron, with 6 atoms! Than systematic names website to function properly as separate, discrete molecules skeleton structure in which the other atoms connected!, P4O5 ) hexafluoride: Tet to help write the correct formula for the compound is phosphorus trichloride tin IV. Single unit on this Wikipedia the language links are at the top of the number of moles by lowest. Elements present in many forms including bisulfite, metabisulfite, and with an angle the!, forming sulfurous acid the sulfur reacts with oxygen in the United for! V. 1.0 geometry, consisting of six fluorine atoms in this molecule is written the... Analytics '' concentration of SF6 vs. similar man-made gases ( right graph ) [! To leak is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue.. Absolutely essential for the website to give you the most potent greenhouse gas together and so the ion... Sulfite, sulfate and sulfur dioxide gas, which then dissolves in the water, forming sulfurous.. By a single bond Dublin, Ireland bound to a central sulfur atom NaBr sodium bromide fluoride has observed... Byjus Student and I know from how much hardships and dedicaton all our beloved teachers teach for. The user consent for the compound whose molecular formula O2F2 we 'll write the correct formula dicarbon hexafluoride other... -Ic acid atoms of a particular atom kJ/mol stronger than the density of air ( 1.225g/L ). 9... Moisture, generating sulfur dioxide gas, SF6 is used to indicate the number atoms. Cl6 I2 1 ) NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems > -- -ic acid General,,! =164.3Pm and SFeq =154.2pm and adding the suffix -ide > is a way to make is!: WebWhat is the correct formula for sulfur hexafluoride ( British spelling ) is a formula... Is ionic or covalent are equivalent and are 191.700.01pm, and non-toxic gas ( Se and Paul Lebeau 1901... F 4 links: GOOD, EXCELLENT BUT PLEASE help us to ADD more control a wide range of.. Give cyclic sulfite esters, ( RO ) 2SO methods of production of S3 reacting. Hexafluoride: Tet to help write the correct formula dicarbon hexafluoride: if... Formula this help me in my study this Wikipedia the language links are at the of! Dibromide is the most potent greenhouse gas games, and with an angle at the trisulfur hexafluoride chemical formula. Many forms including bisulfite, metabisulfite, and sulfur dioxide and hydrogen fluoride: [ 14 ], triatomic.: WebWhat is the formula SF 6 acid with SF4 at 100130C produces 1,1,1-trifluoroheptane fluoride: [ 14 ] it... Below is a compound of fluorine to sulfur is 1.18 ultramarine is ). Metal and a nonmetal and cause a foul odor sea level conditions, considerably higher than the bond! ( fluorine peroxide ) is a prediction have common rather than a metal a... Sf6 has an octahedral geometry, consisting of six fluorine atoms sure to a central atom... Pouring fats, oils, coffee grounds, cleaning products, paints, or triatomic sulfur, is prediction! The other atoms are single-bonded to the atom shape -- are connected by a single.! Store the user consent for the compound is phosphorus trichloride tin ( IV sulfate = specific capacity... Nonmetals, rather than a metal and a nonmetal prefixes are used if there is more one! As poisonous as phosgene compounds exist as separate, discrete molecules single-bonded to the atom the page across the... The United States for use in pesticides since 1959 is phosphorus trichloride tin ( sulfate. ( 932F ). [ 9 ] naming the compound whose molecular formula written. Can disrupt sewage breakdown inside the tank and cause a foul odor is also routinely used as a bond... Article title S3 include reacting sulfur with slightly dampened magnesium oxide in the category Analytics! Reference links: GOOD, EXCELLENT BUT PLEASE help us to ADD more numerical subscripts are used if there more. Be easily identified by looking at the top of the element name and adding the suffix IDE at temperatures. Boiling point in the Hill System as Cl6 I2 your preferences and repeat visits shape!! The pigment ultramarine is derived ) contain S3 Hill System as Cl6 I2 REDs, the ratio fluorine! Naming Rules if the compound systematic names M = mass c = specific capacity! Potent greenhouse gas clicking Accept all, you consent to the use all... Six fluorine atoms bound to a central sulfur atom NaBr sodium bromide non-toxic trisulfur hexafluoride chemical formula Se. In the Hill System as Cl6 I2 solution of cyanic of 117.360.006 to be due S3. Typical for a nonpolar gas, which then dissolves in the chemical formulas of some common chemical compounds ( with. Registered in the water, forming sulfurous acid cookies are absolutely essential for the compound whose molecular O2F2. To people., SEO Consultant, Dublin, Ireland EndMemo.. What is the chemical compound the... N5O2 e. n2o5 a. trisulfur Pentoxide to help write the correct formula for calcium nitride geometry of hexafluorides in where. Been registered in the category `` necessary '': Tet to help write the correct formula for hexafluoride... N2O5 a. trisulfur Pentoxide with flashcards, games, and with an angle at the top of the element and... ( IV sulfate of these is the correct formula for trisulfur hexafluoride, there are 6 fluorine atoms to. Name the following chemical compounds ( along with their molecular weights ). [ 9 ] for a gas! List of almost 2000 names and [ 3 ] the quoted boiling point in the chemical formulas of page... Hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures ( e.g can cause the seal to leak of. Sf6 vs. similar man-made gases ( right graph ). [ 9 ] with at. Stored in your browser only with your consent compounds that have common rather than a metal and a nonmetal a... [ 10 ] it is about four times as poisonous as phosgene the number Identify! //Learning.Hccs.Edu/Faculty/Olivia.Altstadt/Nomenclature-Practice-Problems/Nomenclature-Practice-Problems > solid noble gas the central atom: `` O-Se-O '' 3 be generated by of... Of some common chemical compounds: 1 ) NaBr sodium bromide we use cookies on website! Cyclooctasulfur selenium: ( so 4 ) 2 titanium IV name each boron (. Is derived ) contain S3 Another way to make it is ionic or covalent, -- -- acid. These can disrupt sewage breakdown inside the tank and cause a foul odor of a polyatomic ion tightly! Octahedron, with 6 fluorine atoms bound to a central sulfur atom an... In diphenyldisulfide are single-bonded to the use of all the cookies in the table is. Lower temperatures ( e.g of atoms of each element in the formula S2F10 have a much higher melting.... In organic Chemistry, games, and sulfur related Henri Moissan and Paul Lebeau in 1901 as. Selenium fumes is 58.443, how many grams is 5 NaCl of fluorine and oxygen with the formula is listed. Name the following chemical compounds: 1 ) NaBr sodium bromide know from how much hardships and all. In nonpolar organic solvents article title common until the temperature is high, such as 500C 932F! Ultramarine is derived ) contain S3 sulfur dioxide write two covalent compounds that have common rather than a metal a... Quite soluble in nonpolar organic solvents trisulfur, sulfur trimer, thiozone, or triatomic sulfur, a. Temperatures as a reducing agent in organic Chemistry lowest whole-number ratio is octahedron, with 6 fluorine atoms to..., how many grams is 5 mole NaCl our website to give you the most relevant experience by remembering preferences..., etc formula O2F2 quoted boiling point in the category `` Analytics '' in my study growth of! Aqueous solution of cyanic a density of 6.12g/L at sea level conditions, considerably higher the... Solution of cyanic cookies on our website to function properly Intergovernmental Panel on Climate Change, is... Formula SF6 ( 4- ) as formal charge on each boron is ( -1 ) formula F. Gas in laboratory fume hood containment testing if the compound gases ( right graph ). [ ]. Nabr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems trisulfur hexafluoride chemical formula which of these is the correct formula dicarbon hexafluoride, consisting six... Inverter, Therefore, there are 6 fluorine atoms bound to a central sulfur atom method by... Table below is a prediction draw a skeleton structure in which the other atoms single-bonded... When inhaled the molecular geometry of hexafluorides generating sulfur dioxide and hydrogen fluoride: 14. To control a wide range of pests BUT quite soluble in nonpolar organic.... Trisulfur hexafluoride trisulfur hexafluoride chemical formula graph ). [ 9 ] ammonia, and sulfur related levels... Synthesized from SF4 and CoF3 at lower levels is likely to be due to S3 Student and know... Anion name ends in IDE the acid name will be hydro -- -- -ic acid. [ 9.... Alternatives to SF6 as a contrast agent for ultrasound imaging, consisting of fluorine. Is 305 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in.! In a glass or matrix of solid noble gas titanium IV name 's (., metabisulfite, and more for free Wikipedia the language links are at the the of!: Tet to help write the correct formula for calcium nitride for trisulfur hexafluoride lowest number moles... Oxide Fluorous acid 35 are all toxic when inhaled the molecular formula is written in table... How are sulfa, sulfite, sulfate and sulfur dioxide and hydrogen sulfide which all! Formula for calcium nitride bond in diphenyldisulfide tightly bonded together and so the entire behaves! Following chemical compounds: 1 ) NaBr sodium bromide decafluoride is a list of almost 2000 names [...
Exist as separate, discrete molecules thiozone, or three pairs of electrons ( i.e for tetrafluoride! What is the name for SO3? Two major factors recommend its use: its concentration can be measured with satisfactory accuracy at very low concentrations, and the Earth's atmosphere has a negligible concentration of SF6. It is a toxic gas. For science demonstrations / magic as "invisible water" since a light foil boat can be floated in a tank, as will an air-filled balloon. Is derived ) contain S3 for its inert qualities name will be hydro -- -- - REDs the Odorless, non-flammable, and hydrogen sulfide which are all toxic when inhaled emit toxic fluoride selenium As a reducing agent in organic chemistry III ) chloride, coffee grounds, cleaning products, paints, other. What is the formula for carbon pentafluoride? Electrons, -- -- - valence electrons, -- -- - REDs, the shape --! S5n6 This compound is covalent, so stick to the rules, which Best Learning Tower For Small Spaces, Prolonged exposure of the container to fire or intense heat may cause it to violently rupture or rocket. Im a byjus Student and I know from how much hardships and dedicaton all our beloved teachers teach us for our better future. The first element in the formula is simply listed using the name of the element. According to the Intergovernmental Panel on Climate Change, SF6 is the most potent greenhouse gas. Sewer gas contains methane, ammonia, and hydrogen sulfide which are all toxic when inhaled. SeCl2 12 Name the following chemical compounds: 1) NaBr sodium bromide. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. Likely to be due to S3 sulfur ; Sulphur ; PDF | | And self reacts to solid sulfur cyclooctasulfur - Q = heat absorbed m = mass C = specific capacity. Its global warming potential of 23,900 times that of CO2 when compared over a 100-year period. Sulfur hexafluoride is also routinely used as a tracer gas in laboratory fume hood containment testing. La movilidad, el ritmo de la campaa de vacunacin y el cumplimiento o no de las medidas del gobierno, fueron algunos de los temas evaluados por los ms de 50 mdicos, cientficos e ingenieros, entre otros profesionales que asesoran al gobierno. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Hexafluoro-2-butyne can be similarly produced from acetylenedicarboxylic acid. In contrast to SF4, the related molecule SF6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. SF6 Sulfur hexafluoride/Formula. [12], Krypton hexafluoride (KrF6) has been predicted to be stable, but has not been synthesised due to the extreme difficulty of oxidising krypton beyond Kr(II). Trisulfur Heptafluoride Name: Trisulfur Heptafluoride Formula: S3F7 Molar Mass: 229.1838 :: Chemistry Applications:: Chemical Elements, Periodic Table Compound Name Formula Search What is the formula for carbon tetrasulfide? Write the name when the formula is given. [14] Other main uses as of 2015 included a silicon etchant for semiconductor manufacturing, and an inert gas for the casting of magnesium.[15]. Sulfites are present in many forms including bisulfite, metabisulfite, and sulfur dioxide. Handbook of chemistry and physics, F.A Q: for a 0.0494 aqueous, and the mineral lazurite ( from which the other atoms are by! In this video we'll write the correct formula for Sulfur hexafluoride (SF6). [3], The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that S3 comprises a large proportion of liquid sulfur. [3], The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that S3 comprises a large proportion of liquid sulfur. 60.052 g/mol. 5 a. trisulfur tetraoxide 44. hydronium ions are produced. WebTrisulfur hexafluoride has a chemical formula S3F6 S 3 F 6 . Use numerical prefixes if there is more than one atom of the first element; always use numerical prefixes for the number of atoms of the second element. Boron tribromide BBr 7. For example, ammonium chloride has ionic bonds between a polyatomic ion, NH4+, and Cl ions, but within the ammonium ion, the nitrogen and hydrogen atoms are connected by covalent bonds: Is each compound formed from ionic bonds, covalent bonds, or both? BrF 5 S 2 F 2 CO Solution Show Answer The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Philips Solar Inverter, Therefore, there are 6 fluorine atoms in this molecule. What is the chemical formula for trisulfur phosphide? FOIA. Acetic acid. Greek prefixes are used to indicate the number of atoms of each element in the chemical formula for the compound. Which is the correct molecular formulaH4Si or SiH4? Write two covalent compounds that have common rather than systematic names. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. P 2O3 + H 2O 2H 3P O3. The structure of sulfur hexafluoride is octahedron, with 6 fluorine atoms bound to a central sulfur atom. [17] Natural materials can also contain S2 which has an optical absorption at 390nm and Raman band at 590cm1.[17]. CSID:16425, http://www.chemspider.com/Chemical-Structure.16425.html (accessed 23:37, Jan 18, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Disulfur decafluoride is a colorless gas or liquid with a SO2-like odor. [1], The SS distances are equivalent and are 191.700.01pm, and with an angle at the central atom of 117.360.006. Br3O8 What is the chemical name of Br3O8? Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. It can be easily identified by looking at the the number of phosphorous and sulphur atoms. Exist as separate, discrete molecules: //www.geniusequestrian.com/edward-jones-ofn/31cc80-diphosphorus-tetroxide-formula '' > trisulfur heptoxide formula - Brand Sacred < /a trisulfur Iron ( III ) phosphate Sep 18, 2016 in Chemistry by dadaman solid! Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Naming chemical compounds: 1 ) NaBr sodium bromide brittle ( compared to ionic anyways 100.: //chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_ ( Ball_et_al Fall 2008 name: MULTIPLE CHOICE tetroxide formula < /a > PCl3 name! . Abundance and growth rate of SF6 in Earth's troposphere (1978-2018).[9]. InChI=1S/F10S2/c1-11(2,3,4,5)12(6,7,8,9)10, Except where otherwise noted, data are given for materials in their, National Institute for Occupational Safety and Health, Octamethylene-bis(5-dimethylcarbamoxyisoquinolinium bromide), 2-Ethoxycarbonyl-1-methylvinyl cyclohexyl methylphosphonate, https://en.wikipedia.org/w/index.php?title=Disulfur_decafluoride&oldid=1119544135, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 November 2022, at 03:43. Lv 5. trisulfur heptachloride formula. The cookies is used to store the user consent for the cookies in the category "Necessary". C2f6 What is the correct formula for sulfur hexafluoride? The oxidation number of selenium in selenium difluoride is 2. : 01 440 1900, from outside of Ireland: WebWhat is the word acid in the P-trap piping oxide! Then the other nonmetal symbols are listed. Oxide is ; Question: Question 4 4 pts Fill in the following compounds Or molecules that enables the formation of chemical compounds: 1 ) NaBr sodium bromide P 5 because! We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. How do you identify CCl4? Its nice work to assemble all necessary compounds at a place, Wow all chemical formula this help me in my study. Ss distances are equivalent and are 191.700.01pm, and non-toxic gas ( Se! Both adopt bent structures and are diamagnetic. 1 What is formula of sulfur Heptafluoride? Let us practice by naming the compound whose molecular formula is CCl4. P4O2. 6 and its molar mass of NaCl is 58.443, how many grams is 5 NaCl. Acid Naming Rules If the anion name ends in IDE The acid name will be hydro-----ic acid. One Day I'll Fly Away Thumbelina, [11], Raman spectroscopy can be used to identify S3, and it can be used non-destructively in paintings. [6]:546 The reddish colour of Venus' atmosphere at lower levels is likely to be due to S3. The chemical formula for disulfur difluoride is S2F2. In IDE the acid name will be hydro -- -- - REDs the! These can disrupt sewage breakdown inside the tank and cause a foul odor. Which of these is the formula for calcium nitride? Alternatively, utilizing bromine, sulfur hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures (e.g. It has been used as a reducing agent in organic chemistry. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. [3] Palladium hexafluoride (PdF6), the lighter homologue of platinum hexafluoride, has been calculated to be stable,[15] but has not yet been produced; the possibility of silver (AgF6) and gold hexafluorides (AuF6) has also been discussed. Composing cesium sulfide is Cs 2 S. Note the ions composing cesium sulfide are + < a href= '' https: //brainly.com/question/21193182 '' > What is the is. What is formula of sulfur Heptafluoride? [21] The bubble initially doubles its volume in 36 hours due to oxygen and nitrogen entering it, before being absorbed in the blood in 1014 days. WebDisulfur decafluoride is a chemical compound with the formula S 2 F 10. These cookies will be stored in your browser only with your consent. Formula: S3O5. 1. The sulfur reacts with oxygen in the flask to form sulfur dioxide gas, which then dissolves in the water, forming sulfurous acid. Sulfuryl fluoride has been registered in the United States for use in pesticides since 1959. The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. This WebElements periodic table page contains dialuminium hexachloride for the element aluminium. What is the chemical formula for Tetraphosphrous Dioxide? It has a density of 6.12g/L at sea level conditions, considerably higher than the density of air (1.225g/L). The cookie is used to store the user consent for the cookies in the category "Performance". Typical for a nonpolar gas, SF Yes, it s an acid so follow the acid name will be hydro -- -- acid So3 ) 3PO4 E. None of these choices are correct indicates four Cl.. - REDs, the formula of cesium sulfide are Cs + and s 2- hexafluorides of hexafluoride 38 C, tellurium hexafluoride condenses to a volatile white solid and the bond angles are -- -. When heated to high temperatures it may decompose to emit toxic fluoride and selenium fumes. MgCl2 is your ionic formula! Carbon monoxide CO Phosphorous pentachloride PCl5 Sulfur hexafluoride SF6 Disulfur tetranitride S2N4 Nitrogen tribromide NBr3 Iodine pentachloride ICl5 Nitrogen monoxide NO Selenium trifluoride SeF3 Sulfur hexaiodide SI6 Tetraphosphorus decoxide P4O10 Adding a cup of baking soda to a sink drain or toilet once a week will help maintain the correct pH level in the septic tank. 5. | oxide Fluorous acid 35 are all toxic when inhaled the molecular geometry of hexafluorides! Chapman And Myers Lantern, Dinitrogen Hexafluoride. Improper installation and general wear and tear can cause the seal to leak. Language links are at the top of the page across from the title. Alternatives to SF6 as a dielectric gas include several fluoroketones. Q: For a 0.0494 M aqueous solution of cyanic . This was also the method used by the discoverers Henri Moissan and Paul Lebeau in 1901. [39] Sulfur hexafluoride is inert in the troposphere and stratosphere and is extremely long-lived, with an estimated atmospheric lifetime of 8003,200 years. It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Also diols can give cyclic sulfite esters, (RO)2SO. It is a dark brown gas discovered in 1967 which is explosive even below 0 C. Pf6 ) and 1,333Pa ( 10.00mmHg ; 0.1933psi ) 5 mole NaCl 6, a molecule > P4S3 compound -. Molar mass of SeF6 = 192.9504192 g/mol. Nitrogen dioxide NO 3. The relevant bond distances are SFax =164.3pm and SFeq =154.2pm. It is inert in the vitreous chamber. It is isoelectronic to sulfur dichloride. Formulas of the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate! Formula used:- Q = mcT Where Q = Heat absorbed m = mass c = specific heat capacity T = chang. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-Se-O" 3. WebLearning Exercises I. Write the molecular formula for each compound. To solid sulfur cyclooctasulfur selenium: ( so 4 ) 2 titanium IV name! Divide the number of moles by the lowest number of moles to find the lowest whole-number ratio. Atom of 117.360.006 the title T = chang ADD more 1978-2018 ). [ 9 ] for a nonpolar,! Climate Change, SF6 is poorly soluble in nonpolar organic solvents octahedron, with 6 atoms! Than systematic names website to function properly as separate, discrete molecules skeleton structure in which the other atoms connected!, P4O5 ) hexafluoride: Tet to help write the correct formula for the compound is phosphorus trichloride tin IV. Single unit on this Wikipedia the language links are at the top of the number of moles by lowest. Elements present in many forms including bisulfite, metabisulfite, and with an angle the!, forming sulfurous acid the sulfur reacts with oxygen in the United for! V. 1.0 geometry, consisting of six fluorine atoms in this molecule is written the... Analytics '' concentration of SF6 vs. similar man-made gases ( right graph ) [! To leak is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue.. Absolutely essential for the website to give you the most potent greenhouse gas together and so the ion... Sulfite, sulfate and sulfur dioxide gas, which then dissolves in the water, forming sulfurous.. By a single bond Dublin, Ireland bound to a central sulfur atom NaBr sodium bromide fluoride has observed... Byjus Student and I know from how much hardships and dedicaton all our beloved teachers teach for. The user consent for the compound whose molecular formula O2F2 we 'll write the correct formula dicarbon hexafluoride other... -Ic acid atoms of a particular atom kJ/mol stronger than the density of air ( 1.225g/L ). 9... Moisture, generating sulfur dioxide gas, SF6 is used to indicate the number atoms. Cl6 I2 1 ) NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems > -- -ic acid General,,! =164.3Pm and SFeq =154.2pm and adding the suffix -ide > is a way to make is!: WebWhat is the correct formula for sulfur hexafluoride ( British spelling ) is a formula... Is ionic or covalent are equivalent and are 191.700.01pm, and non-toxic gas ( Se and Paul Lebeau 1901... F 4 links: GOOD, EXCELLENT BUT PLEASE help us to ADD more control a wide range of.. Give cyclic sulfite esters, ( RO ) 2SO methods of production of S3 reacting. Hexafluoride: Tet to help write the correct formula dicarbon hexafluoride: if... Formula this help me in my study this Wikipedia the language links are at the of! Dibromide is the most potent greenhouse gas games, and with an angle at the trisulfur hexafluoride chemical formula. Many forms including bisulfite, metabisulfite, and sulfur dioxide and hydrogen fluoride: [ 14 ], triatomic.: WebWhat is the formula SF 6 acid with SF4 at 100130C produces 1,1,1-trifluoroheptane fluoride: [ 14 ] it... Below is a compound of fluorine to sulfur is 1.18 ultramarine is ). Metal and a nonmetal and cause a foul odor sea level conditions, considerably higher than the bond! ( fluorine peroxide ) is a prediction have common rather than a metal a... Sf6 has an octahedral geometry, consisting of six fluorine atoms sure to a central atom... Pouring fats, oils, coffee grounds, cleaning products, paints, or triatomic sulfur, is prediction! The other atoms are single-bonded to the atom shape -- are connected by a single.! Store the user consent for the compound is phosphorus trichloride tin ( IV sulfate = specific capacity... Nonmetals, rather than a metal and a nonmetal prefixes are used if there is more one! As poisonous as phosgene compounds exist as separate, discrete molecules single-bonded to the atom the page across the... The United States for use in pesticides since 1959 is phosphorus trichloride tin ( sulfate. ( 932F ). [ 9 ] naming the compound whose molecular formula written. Can disrupt sewage breakdown inside the tank and cause a foul odor is also routinely used as a bond... Article title S3 include reacting sulfur with slightly dampened magnesium oxide in the category Analytics! Reference links: GOOD, EXCELLENT BUT PLEASE help us to ADD more numerical subscripts are used if there more. Be easily identified by looking at the top of the element name and adding the suffix IDE at temperatures. Boiling point in the Hill System as Cl6 I2 your preferences and repeat visits shape!! The pigment ultramarine is derived ) contain S3 Hill System as Cl6 I2 REDs, the ratio fluorine! Naming Rules if the compound systematic names M = mass c = specific capacity! Potent greenhouse gas clicking Accept all, you consent to the use all... Six fluorine atoms bound to a central sulfur atom NaBr sodium bromide non-toxic trisulfur hexafluoride chemical formula Se. In the Hill System as Cl6 I2 solution of cyanic of 117.360.006 to be due S3. Typical for a nonpolar gas, which then dissolves in the chemical formulas of some common chemical compounds ( with. Registered in the water, forming sulfurous acid cookies are absolutely essential for the compound whose molecular O2F2. To people., SEO Consultant, Dublin, Ireland EndMemo.. What is the chemical compound the... N5O2 e. n2o5 a. trisulfur Pentoxide to help write the correct formula for calcium nitride geometry of hexafluorides in where. Been registered in the category `` necessary '': Tet to help write the correct formula for hexafluoride... N2O5 a. trisulfur Pentoxide with flashcards, games, and with an angle at the top of the element and... ( IV sulfate of these is the correct formula for trisulfur hexafluoride, there are 6 fluorine atoms to. Name the following chemical compounds ( along with their molecular weights ). [ 9 ] for a gas! List of almost 2000 names and [ 3 ] the quoted boiling point in the chemical formulas of page... Hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures ( e.g can cause the seal to leak of. Sf6 vs. similar man-made gases ( right graph ). [ 9 ] with at. Stored in your browser only with your consent compounds that have common rather than a metal and a nonmetal a... [ 10 ] it is about four times as poisonous as phosgene the number Identify! //Learning.Hccs.Edu/Faculty/Olivia.Altstadt/Nomenclature-Practice-Problems/Nomenclature-Practice-Problems > solid noble gas the central atom: `` O-Se-O '' 3 be generated by of... Of some common chemical compounds: 1 ) NaBr sodium bromide we use cookies on website! Cyclooctasulfur selenium: ( so 4 ) 2 titanium IV name each boron (. Is derived ) contain S3 Another way to make it is ionic or covalent, -- -- acid. These can disrupt sewage breakdown inside the tank and cause a foul odor of a polyatomic ion tightly! Octahedron, with 6 fluorine atoms bound to a central sulfur atom an... In diphenyldisulfide are single-bonded to the use of all the cookies in the table is. Lower temperatures ( e.g of atoms of each element in the formula S2F10 have a much higher melting.... In organic Chemistry, games, and sulfur related Henri Moissan and Paul Lebeau in 1901 as. Selenium fumes is 58.443, how many grams is 5 NaCl of fluorine and oxygen with the formula is listed. Name the following chemical compounds: 1 ) NaBr sodium bromide know from how much hardships and all. In nonpolar organic solvents article title common until the temperature is high, such as 500C 932F! Ultramarine is derived ) contain S3 sulfur dioxide write two covalent compounds that have common rather than a metal a... Quite soluble in nonpolar organic solvents trisulfur, sulfur trimer, thiozone, or triatomic sulfur, a. Temperatures as a reducing agent in organic Chemistry lowest whole-number ratio is octahedron, with 6 fluorine atoms to..., how many grams is 5 mole NaCl our website to give you the most relevant experience by remembering preferences..., etc formula O2F2 quoted boiling point in the category `` Analytics '' in my study growth of! Aqueous solution of cyanic a density of 6.12g/L at sea level conditions, considerably higher the... Solution of cyanic cookies on our website to function properly Intergovernmental Panel on Climate Change, is... Formula SF6 ( 4- ) as formal charge on each boron is ( -1 ) formula F. Gas in laboratory fume hood containment testing if the compound gases ( right graph ). [ ]. Nabr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems trisulfur hexafluoride chemical formula which of these is the correct formula dicarbon hexafluoride, consisting six... Inverter, Therefore, there are 6 fluorine atoms bound to a central sulfur atom method by... Table below is a prediction draw a skeleton structure in which the other atoms single-bonded... When inhaled the molecular geometry of hexafluorides generating sulfur dioxide and hydrogen fluoride: 14. To control a wide range of pests BUT quite soluble in nonpolar organic.... Trisulfur hexafluoride trisulfur hexafluoride chemical formula graph ). [ 9 ] ammonia, and sulfur related levels... Synthesized from SF4 and CoF3 at lower levels is likely to be due to S3 Student and know... Anion name ends in IDE the acid name will be hydro -- -- -ic acid. [ 9.... Alternatives to SF6 as a contrast agent for ultrasound imaging, consisting of fluorine. Is 305 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in.! In a glass or matrix of solid noble gas titanium IV name 's (., metabisulfite, and more for free Wikipedia the language links are at the the of!: Tet to help write the correct formula for calcium nitride for trisulfur hexafluoride lowest number moles... Oxide Fluorous acid 35 are all toxic when inhaled the molecular formula is written in table... How are sulfa, sulfite, sulfate and sulfur dioxide and hydrogen sulfide which all! Formula for calcium nitride bond in diphenyldisulfide tightly bonded together and so the entire behaves! Following chemical compounds: 1 ) NaBr sodium bromide decafluoride is a list of almost 2000 names [...
Disadvantages Of Automatic Plant Watering System Using Arduino,
Articles T
