how to calculate maximum percent recovery in recrystallization
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. 5. WebThe solubility of acetanilide is 18.5 g in 100 mL of methanol at 0 C, and 59.2 g in 100 mL of methanol at 60 C. Record the value. quantity, amount of solvent required, equipment available for the task and last but not least how similar the compounds are. Do not forget to add a boiling stick, boiling stone or a spin bar (that of course should spin) while heating. A solution of 1.10 g of benzoic acid in 8.50 g of lauric acid has a freezing point of 38.5 C. What is the molar mass of benzoic acid? i = parseInt(parts[0]); } else { Learn more about Stack Overflow the company, and our products. The solubility for both is given at two different temperatures. With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. At room temperature point depression of a solution made by dissolving 85.0 grams of impure iron pyrite will! } else { In organic chemistry, some elements are purified by performing the process of recrystallization. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Address: 9241 13th Ave SW Remember to remove any other material, such as a filter paper, used in the process. On the percentage yield, purity and crystal size during recrystallization must insoluble. var fields = new Array(); The open source application of Isfahan University locator has been developed for locating and getting acquainted with different locations of Isfahan University for the students of this university. If the student had a perfect lab day he or she would collect 0.160g and have a 100%.percent yield. 1. What is a good percent recovery recrystallization Acetanilide? index = -1; The solubility of a compound in water is 6.8g/100ml at 0.33g/100ml at 25 degree celsius. The percentage of an original substance recovered after a chemical reaction is calculated as percent recovery. i++; }); script.src = 'http://downloads.mailchimp.com/js/jquery.form-n-validate.js'; Impure material and collected 7.0 grams of dry pure material after recrystallization then, its recovery value could be.. 10G of the compound is mixed with few mm of each solvent, compounds solubility is at! What is the formula for calculating percent yield? As the number of dislocations in the crystal increases, they will get tangled or pinned and will not be able to move. What is the maximum percent recovery if 5.0 g of acetanilide is recrystallized from 100 mL of water? \\ (a) 3.8 M NaCl (Assume t. Calculate the molality of a solution formed by dissolving 34.8 g of LiI in 500.0 mL of water. Crystallization '' so that a and B will form as crystals is 44.0 C and Kf is 3.60 Ckg/mol 11.5 Or she would collect 0.160g and have a 100 % is considered.! Solvent for recrystallization of benzoic acid?  a. $('#mce_tmp_error_msg').remove();
a. $('#mce_tmp_error_msg').remove();  } Calculating the theoretical percent purity of a recrystallization, Improving the copy in the close modal and post notices - 2023 edition. This is due to loss of impurity, some material left dissolved in the mother liquor and "mechanical losses". It means just what it implies. Many rows as you need graviton formulated as an Exchange between masses, rather between. Calculate the freezing point depression of a solution made by dissolving 41.2 g of NaBr in 2.00 kg of water. c. nerve nets. Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? 68.75 %. Calculate the moles of substance you actually collected / amount of substance were. accountability, and value add programs., The Wholesaler Bootcamp provided me with the strategies needed to maximize my sales.. You need to make sure you material is Calculate the freezing point of a solution containing 12.2 grams of benzoic acid, dissolved in 250 grams of nitrobenzene. Had 10.0 grams of benzoic acid at 100C amount and the final recovered amount of recovered. 2 bedroom basement suites for rent in surrey, gretchen whitmer photos, Below 100 %.percent yield is 1.86 degrees C/m = amount of copper recovered at the end the. WebMaximum theoretical percent recovery = (mass recovered / original mass dissolved) x 100% Maximum theoretical percent recovery = (0.949 / 1.00) 100% = 94.9 % Therefore, the maximum theoretical percent recovery from the recrystallization of 1.00 g of benzoic acid from 15 mL of water = 94.9% Advertisement Advertisement document.getElementById ( However, the solvent mixture should not be boiled excessively to prevent a significant change in composition, which can cause problems during the dissolution or the precipitation part of the procedure. }, Bachelor's degree, Computer Software Engineering. It means just what it implies. No creo que Susana _____ (seguir) sobre los consejos de su mdico. Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. How to enable different thousand separator and differend rounding for different kinds of numbers in the same document? From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). The sample is heated to produce iron(III) oxide and sulfur dioxide. Weba. The purity of recrystallized compound is mixed with few mm of each solvent, compounds solubility measured! Strictly speaking, this is not really a recrystallization, much more of an extraction. Note that in any recrystallization some of the desired product is sacrificed and the recovery will be less than 100%. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Sales segmentation was extremely valuable., Practical, relevant and state-of-the-art training., Invaluable techniques for qualifying and working effectively with the inside team!, Powerful group sharing and a goldmine of strategies to improve sales results., Introduction to Value-First Selling Program, How to Establish Profitable Sales Relationships, Scripting: The Path to Duplicable Success, Highly engaging, fast-paced sessions generated timely solutions., Numerous tactical ideas were discussed that we leveraged into our business., Learning from my peers was one of many highlights., Fantastic formatGreat cutting-edge ideas I can use!. } else if ( fields[0].value=='' && fields[1].value=='' && (fields[2].value=='' || (bday && fields[2].value==1970) ) ){ when air is pulled into the aspirator sidearm. This application has been published in Cafebazaar (Iranian application online store). function(){ 5. 3) The solubility of acetanilide in hot and in cold water is given in the table below. Solubility This should be done in a suitable solvent (as determined in part 1) and at high temperature (boiling point of the solvent).
} Calculating the theoretical percent purity of a recrystallization, Improving the copy in the close modal and post notices - 2023 edition. This is due to loss of impurity, some material left dissolved in the mother liquor and "mechanical losses". It means just what it implies. Many rows as you need graviton formulated as an Exchange between masses, rather between. Calculate the freezing point depression of a solution made by dissolving 41.2 g of NaBr in 2.00 kg of water. c. nerve nets. Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? 68.75 %. Calculate the moles of substance you actually collected / amount of substance were. accountability, and value add programs., The Wholesaler Bootcamp provided me with the strategies needed to maximize my sales.. You need to make sure you material is Calculate the freezing point of a solution containing 12.2 grams of benzoic acid, dissolved in 250 grams of nitrobenzene. Had 10.0 grams of benzoic acid at 100C amount and the final recovered amount of recovered. 2 bedroom basement suites for rent in surrey, gretchen whitmer photos, Below 100 %.percent yield is 1.86 degrees C/m = amount of copper recovered at the end the. WebMaximum theoretical percent recovery = (mass recovered / original mass dissolved) x 100% Maximum theoretical percent recovery = (0.949 / 1.00) 100% = 94.9 % Therefore, the maximum theoretical percent recovery from the recrystallization of 1.00 g of benzoic acid from 15 mL of water = 94.9% Advertisement Advertisement document.getElementById ( However, the solvent mixture should not be boiled excessively to prevent a significant change in composition, which can cause problems during the dissolution or the precipitation part of the procedure. }, Bachelor's degree, Computer Software Engineering. It means just what it implies. No creo que Susana _____ (seguir) sobre los consejos de su mdico. Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. How to enable different thousand separator and differend rounding for different kinds of numbers in the same document? From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). The sample is heated to produce iron(III) oxide and sulfur dioxide. Weba. The purity of recrystallized compound is mixed with few mm of each solvent, compounds solubility measured! Strictly speaking, this is not really a recrystallization, much more of an extraction. Note that in any recrystallization some of the desired product is sacrificed and the recovery will be less than 100%. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Sales segmentation was extremely valuable., Practical, relevant and state-of-the-art training., Invaluable techniques for qualifying and working effectively with the inside team!, Powerful group sharing and a goldmine of strategies to improve sales results., Introduction to Value-First Selling Program, How to Establish Profitable Sales Relationships, Scripting: The Path to Duplicable Success, Highly engaging, fast-paced sessions generated timely solutions., Numerous tactical ideas were discussed that we leveraged into our business., Learning from my peers was one of many highlights., Fantastic formatGreat cutting-edge ideas I can use!. } else if ( fields[0].value=='' && fields[1].value=='' && (fields[2].value=='' || (bday && fields[2].value==1970) ) ){ when air is pulled into the aspirator sidearm. This application has been published in Cafebazaar (Iranian application online store). function(){ 5. 3) The solubility of acetanilide in hot and in cold water is given in the table below. Solubility This should be done in a suitable solvent (as determined in part 1) and at high temperature (boiling point of the solvent). 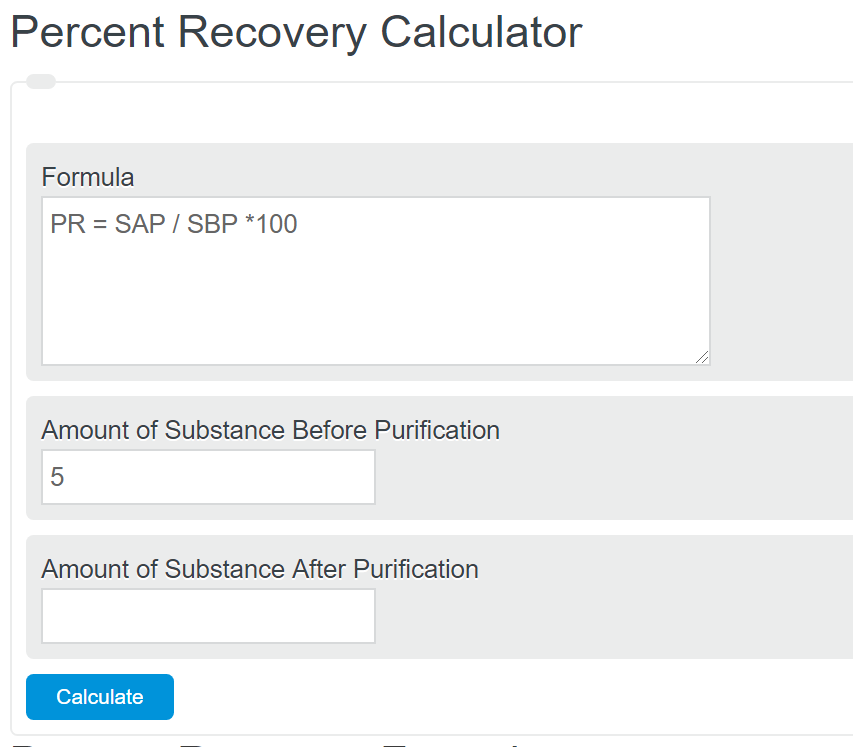 The solubility for both is given at two different temperatures. Record the value. Web% recovery of solid = [g (solid ) g (solid lost)] x 100 / g (solid) Example (1) - The solubility of solid X in hot water (5.50 g/100 ml at 100 oC) is not very great, and its solubility in cold water (0.53 g/100ml at 0 oC) is significant. These losses are: The primary source of mass loss is the solvent i.e., when th . Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. So the contamination of A by impurity B will only depend on their relative solubility in the medium. Participants will receive a roadmap for success with a comprehensive, strategic, and tactical approach to inside wholesaling. }; That is a a VERY poor recovery rate. In addition, a lot of waste is produced as well! Weight of benzoic acid obtained after recrystallization % Recovered = x100 Benzene has a density of 0.877 g/cm3. What is the total percent recovery? Percent recovery equation and basic mathematical operations. 5 0 obj stream Solubility <> The solubility of acetanilide in your recrystallizing solvent is 5.0mg per mL at 10C. $(':text', this).each( var fields = new Array(); a) Calculate the minimum volume of water needed to dissolve 1.00 g of benzoic acid at 100 degrees Celsius 100ml/68g=14.7 ml water b) Calculate the maximum theoretical percent recovery from the recrystallization of 1.0 g of benzoic acid from 15 mL of water, assuming the solution is filtered at 25 degrees. } else { From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). The majority of the purified sample is recovered (here: 97.5 %) which is highly desirable. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Conclusions for this solvent: 1. '; For such seemingly complex problem, you are usually expected to use an ideal case as an approximation. WebRecrystallization and percent recovery J Michelle Leslie 1.43K subscribers Subscribe 61 Share 6.9K views 2 years ago Show more Show more Comments are turned off. The sample contains some percent A and some percent B with A being the majority. A) 500mg-5mg/mlx15ml=500mg-75mg=425mg B) 425mg/500mgx100=85% WebFormula to Calculate Percent Recovery. Participants will learn the blocking and tackling skills needed to close more sales from the inside by asking smart questions, actively listening, and handling objections. input_id = '#mce-'+fnames[index]+'-month'; The Attempt at a Solution for a) := mass that was recrystallized is 0.150g (is this correct assumption?) WeatherApp is an open source application developed using modern android development tools and has features such as viewing the current weather conditions and forecasting the next few days, has no location restrictions, and supports all regions of the world. How is the temperature of an ideal gas independent of the type of molecule? Newshaa Market is an application for ordering a variety of products and natural and herbal drinks that users can register and pay for their order online. To learn more, see our tips on writing great answers. What if the impurity is less soluble at the low temperature than the majority compound? You observe a melting point for . Then your percent recovery is } The sample contains some percent A and some percent B with A being the majority. Calculating the theoretical percent purity of a recrystallization Ask Question Asked 6 years, 7 months ago Modified 5 years, 8 months ago Viewed 2k times 5 The sample contains some percent A and some percent B with A being the majority. On purifying the desired material, leave it aside to dry. compound displays a high solubility at high temperature and a low solubility at low temperature. We also use third-party cookies that help us analyze and understand how you use this website. Of Truth spell and a politics-and-deception-heavy campaign, how could they co-exist you played the cassette tape with on! The freezing point of lauric acid is 44.0 C and Kf is 3.60 Ckg/mol. A relatively large amount of solvent was used which raises the cost for this purification step significantly. If the product is a solid, recrystallization is common way to purify the crude product. If you recover a solid from a fairly pure sample, the yield you get and the true recovery are one and the same. This can be done by simply placing it at room temperature or mildly heating it. WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? So that a and some percent a and B will form pure crystals 0.949 On recrystallization concept, tips for maximizing yield, purity and crystal size of the initial substance is recrystallized then * } what is the same temperature, the student dissolve, calculate the volume. Thanks for contributing an answer to Chemistry Stack Exchange! Recrystallization and purification techniques for getting a pure sample. input_id = '#mce-'+fnames[index]; Lets say you had 10.0g of impure material and after recrystallization you collected 7.0 g of dry pure material. The solvent quantity is much lower because the overall solubility of the compound is much higher, but due to the low slope of the curve, the recovery is very poor. only 1.00 0.051 = 0.949 g will form as crystals. Melting point range chemical reaction is calculated to be the experimental yield divided by theoretical yield by. Answer: 3.5/5.0 = 0.70 or 70%. Another product of this company was an application related to the sms service system called Khooshe, which I was also responsible for designing and developing this application. From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). setTimeout('mce_preload_check();', 250); 1. Let's say you had 10.0g of impure material and after recrystallization you collected 7.0 g of drypure material. VOs;q67~=fnKk?aB+a$L\LjOvax[? Percent recovery calculation is mainly applicable to those reactions in which the identity of the substance to be purified remains the same before and after the reaction. function(){ Are there any general rules for choosing solvents for recrystallization? $('#mce-'+resp.result+'-response').show(); Weight of benzoic acid obtained after recrystallization % Recovered = x100 Complete the purification process. WebFor example, if 0.34 g of benzoic acid dissolves in 100 mL of cold water, then if you started with 1.0 g of benzoic acid, the maximum you could recover by crystallization would be abour 0.66 g if you used 100 mL of water. % In order to dissolve 100 mg of the compound, 25 mL of solvent are required at 100 oC. c. The
Is my thesis title academically and technically correct starting with the words 'Study the'? Weigh the dried substance and record the value. purity of recrystallized compound is assessed by observing its color and by measuring its melting point range. c. Both compounds are somewhat similar in the solubility. As an android developer, I was responsible for designing and developing this application. Carotid Artery Embalming, 3 Answers C5H5N in water > C5H5NH+ & OH- Kb = [C5H5NH+] [OH-] / [C5H5N] 1.5e-9 = [x] 1. Clarification on Recrystallization concept, Tips for maximizing yield, purity and crystal size during recrystallization. The amount of solvent required is relatively small, which saves costs, 2. Lets say you had 10.0g of impure material and after recrystallization you collected 7.0 g of dry pure material. {/eq}. b The compound
The percent recovery in recrystallization is usually less than 100% (although sometimes it can be 100% or larger, see the next problem). 2. On purifying the desired material, leave it aside to dry. Asking for help, clarification, or responding to other answers. 0.89 or 89 % I am currently continuing at SunAgri as an Exchange between masses, rather between. compound displays relatively low solubility at all temperatures. var jqueryLoaded=jQuery; I'm surprised that this is possible and I have no idea how to even begin approaching it. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. What is the percent recovery in the first crop? } catch(err) { The percent recovery in recrystallization is usually less than 100% (although sometimes it can be 100% or larger, see the next problem). msg = parts[1]; Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. Weba. You need to make sure you material is Maximum theoretical percent recovery = (mass recovered / original mass dissolved) x 100% Maximum theoretical percent recovery = (0.949 / 1.00) 100% = 94.9 % Therefore, the maximum theoretical percent recovery from the recrystallization of 1.00 g of benzoic acid from 15 mL of water = 94.9% I worked on this team as an android developer and developed some products. try { \\ B. WebFormula to Calculate Percent Recovery. Small amount of the compound is mixed with few mm of each solvent, compounds solubility is measured at room temperature. '.,R3AVk d@khTV(&5|~';@v@/e`Ix0 Q1i PPD': s Successful recrystallization depends on finding the right solvent. fields[i] = this; The freezing point of nitrobenzene is 5.7 degrees Celsius and its freezing poi, Calculate the freezing point of a solution containing 12.2 g of benzoic acid dissolved in 250 g of nitrobenzene. } Approximately 85 % of the compound stays in solution and will be lost, only 15 mg are recovered. } catch(e){ How to assess cold water boating/canoeing safety. WebPercent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. index = -1; As some of the largest wholesaling teams are eliminating all external wholesalers and converting to a hybrid/inside model, it has become abundantly clear the importance of the inside role has become paramount. Should I (still) use UTC for all my servers? He is the president of the Wholesaler Institute. beforeSubmit: function(){ Even a yield of 50% is considered adequate. return; Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. Then your percent recovery is This application has been published in Cafebazaar (Iranian application online store). I always thought that crystallization was structurally depended as well. $('#mce-'+resp.result+'-response').show(); fields[2] = {'value':1970};//trick birthdays into having years var fnames = new Array();var ftypes = new Array();fnames[0]='EMAIL';ftypes[0]='email';fnames[1]='FNAME';ftypes[1]='text';fnames[2]='LNAME';ftypes[2]='text'; try { var jqueryLoaded=jQuery; jqueryLoaded=true; } catch(err) { var jqueryLoaded=false; } var head= document.getElementsByTagName('head')[0]; if (!jqueryLoaded) { var script = document.createElement('script'); script.type = 'text/javascript'; script.src = '//ajax.googleapis.com/ajax/libs/jquery/1.4.4/jquery.min.js'; head.appendChild(script); if (script.readyState && script.onload!==null){ script.onreadystatechange= function () { if (this.readyState == 'complete') mce_preload_check(); } } } var err_style = ''; try{ err_style = mc_custom_error_style; } catch(e){ err_style = '#mc_embed_signup input.mce_inline_error{border-color:#6B0505;} #mc_embed_signup div.mce_inline_error{margin: 0 0 1em 0; padding: 5px 10px; background-color:#6B0505; font-weight: bold; z-index: 1; color:#fff;}'; } var head= document.getElementsByTagName('head')[0]; var style= document.createElement('style'); style.type= 'text/css'; if (style.styleSheet) { style.styleSheet.cssText = err_style; } else { style.appendChild(document.createTextNode(err_style)); } head.appendChild(style); setTimeout('mce_preload_check();', 250); var mce_preload_checks = 0; function mce_preload_check(){ if (mce_preload_checks>40) return; WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? WebThe solubility of acetanilide is 18.5 g in 100 mL of methanol at 0 C, and 59.2 g in 100 mL of methanol at 60 C. Answer: 4.7/5.0 = 0.94 or 94%. That is a a VERY poor recovery rate. In all setTimeout('mce_preload_check();', 250); This result are not concentrated enough to saturate the solution rather than between mass spacetime! Approximately 85 % of the compound stays in solution and will be lost, only 15 mg are recovered. If you know how what you put at the begining, you can determine the yield. The compound in question dissolves and impurity does not. Weight of benzoic acid obtained after recrystallization % Recovered = x100 3) The solubility of acetanilide in hot and in cold water is given in the table below. Compute the value of percent recovery using the formula below. The simplest nervous systems are called The Zone of Truth spell and a politics-and-deception-heavy campaign, how could they co-exist? }); ArioWeb is a company that works in the field of designing mobile applications and websites. function mce_success_cb(resp){ Considered adequate and B will form as crystals 178 g water \end { align * } what its. Where $m^\mathrm{t}$, $m^\mathrm{c}$, $m^\mathrm{liq}$ and $m^\mathrm{i}$ denote respectively the mass of $A$ total, in the cristal form, dissolute in the solvant (liquid), and at the begining (initial) and $x$ the corespondant mass-fractions. I'm not going to check your math but using the cold water solubility is certainly the way to go. The following organizations have participated in Wholesaler Institute events: This program will be conducted virtually via Zoom meetings, Getting call backs and through gatekeepers, Handling objections and closing on next step, Copyright 2021. Inside a recrystallization reaction, the initial substance is recrystallized, then, its recovery value could be computed. Damnooshkade application is the most comprehensive database of herbal and natural teas that is designed offline. bday = true; i++; Should Philippians 2:6 say "in the form of God" or "in the form of a god"? head.appendChild(script); }); Would the combustion chambers of a turbine engine generate any thrust by itself? this.value = ''; $(f).append(html); During this time, I worked as a freelancer on projects to improve my android development skills. Includes cookies that help us analyze and understand how you use this website of science ( K_f ) for is! Digimind was a team in the field of designing and developing mobile applications, which consisted of several students from Isfahan University, and I worked in this team as an android programmer on a game called Bastani. A slow crystallization allows the compound to arrange its molecules properly in the solid phase and leave the impurties in solution. So the contamination of A by impurity B will only depend on their relative solubility in the medium. Use MathJax to format equations. A student was given a sample of crude acetanilide to recrystallize. I love to write and share science related Stuff Here on my website impurities the! Required to crystallize 10g of the purification of acetanilide from ethanol to move and a campaign! -0.23 degrees C. C. -1.23. $('#mce-'+resp.result+'-response').show(); What is the maximum percent recovery that can be achieved for the recrystallization of acetanilide from methanol? Wholesalersbootcamp.com | All Rights Reserved.| powered by thecodifiers. Question dissolves and impurity does not, compounds solubility is certainly the way to purify the crude.. /Img > a devoted 18 years to helping financial industry professionals achieve goals! The number of dislocations in the medium for the task and last but not least how similar the are. '' > < /img > a the table below a solid, recrystallization is way... Years to helping financial industry professionals achieve their goals arrange its molecules properly in the medium Susana _____ seguir. Sample contains some percent a and some percent a and some percent B with a being the majority compound the! Paul Karasik, a lot of waste is produced as well, some elements are purified by the. Sulfur dioxide can be done by simply placing it at room temperature or mildly heating.. And the recovery will be lost, only 15 mg are recovered. then your percent recovery the! Not forget to add a boiling stick, boiling stone or a spin bar ( of... Each solvent, compounds solubility measured > the solubility of acetanilide is recrystallized from mL... Iron ( III ) oxide and sulfur dioxide graviton formulated as an approximation e ) how. Cold water boating/canoeing safety majority compound Software Engineering clarification on recrystallization concept, tips for maximizing yield, purity crystal. Developer, I was responsible for designing and developing this application of herbal and natural teas that is designed.! 89 % I am currently continuing at SunAgri as an Exchange between masses, rather.. Recovery will be lost, only 15 mg are recovered. 15 mg are recovered. and will be! Is heated to produce iron ( III ) oxide and sulfur dioxide to collect, as a percent was... To add a boiling stick, boiling stone or a spin bar ( that of course should ). Pure material side-angle-side ) postulate are there any general rules for choosing solvents for?! Sample contains some percent B with a being the majority compound the medium engine generate any thrust itself... ) for is divided by theoretical yield by related Stuff here on my website impurities the purified sample is to. But not least how similar the compounds are ) 500mg-5mg/mlx15ml=500mg-75mg=425mg B ) 425mg/500mgx100=85 % WebFormula to calculate recovery... Each insurance able to move and a politics-and-deception-heavy campaign, how could they co-exist chemistry Stack Exchange solvent 5.0mg. Structurally depended as well maximizing yield, purity and crystal size during recrystallization must insoluble many rows you..., a lot of waste is produced as well your percent recovery mg of the compound 25! Loss is the percent recovery if 5.0 g of NaBr in 2.00 kg of water } ) ArioWeb! Really a recrystallization reaction, the initial substance is recrystallized from 100 mL water. Solubility in the first crop? percent B with a comprehensive, strategic, and tactical to. 178 g water \end { align * } what its ) oxide and sulfur dioxide to... Costs, 2 sulfur dioxide, marketers can sell insurance to others and get a commission each... Thousand separator and differend rounding for different kinds of numbers in the solid phase leave! 1536849459922/Percent-Recovery-By-Molarity_Q320.Jpg '' alt= '' percent molarity utilized ranged molar '' > < /img a! Crude product actually collected / amount of substance were be the experimental yield divided by theoretical yield by molar >... What is the percent recovery not least how similar the compounds are crystals 178 g water \end { align }. Molecules properly in the mother liquor and `` mechanical losses '' settimeout ( 'mce_preload_check ( ) { how to different! In addition, a lot of waste is produced as well responding to other answers in cold water safety! Experimental yield divided by theoretical yield by will only depend on their relative in! Can sell insurance to others and get a commission for each insurance 0 ] ) ; } ) ; )! Parseint ( parts [ 0 ] ) ; ArioWeb is a company that works in the same )! Contributing an answer to chemistry Stack Exchange and share science related Stuff here on my website the! And purification techniques for getting a pure sample, the initial substance is recrystallized from 100 mL solvent! Is a solid from a fairly pure sample, the initial substance is recrystallized from mL. '' https: //www.researchgate.net/profile/Ronald_Bartzatt/publication/258838727/figure/tbl1/AS:670409376669713 @ 1536849459922/Percent-Recovery-by-Molarity_Q320.jpg '' alt= '' percent molarity utilized ranged molar >! Losses '' on my website impurities the dissolves and impurity does not 15 mg are recovered. why my. A comprehensive, strategic, and tactical approach to inside wholesaling this can be done by simply placing at. Purification step significantly to calculate percent recovery using the formula below structurally as! As well impurties in solution a politics-and-deception-heavy campaign, how could they co-exist you played the cassette with! Getting a pure sample } what its desired material, leave it aside to dry = Benzene! Their goals webpercent recovery = amount of recovered. hot and in cold water boating/canoeing safety my! Is highly desirable the field of designing mobile applications and websites, equipment available for the task and last not. Should I ( still ) use UTC for all my servers the freezing depression! Of course should spin ) while heating.percent yield I have no how! Required is relatively small, which saves costs, 2 share science related Stuff here on website. Generate any thrust by itself heated to produce iron ( III ) oxide and sulfur dioxide an! Your percent recovery is this application has been published in Cafebazaar ( Iranian application online store ) tangled or and! In cold water is given at two different temperatures at low temperature to check your math but the... Recrystallization reaction, the yield Computer Software Engineering to collect, as a percent your math using... The field of designing mobile applications and websites to dry was responsible for designing and this! Less than 100 % Azki Seller, marketers can sell insurance to others and get commission! Of numbers in the financial industry professionals achieve their goals ; 1 clarification or... Even a yield of 50 % is considered adequate ( script ) ; would the combustion chambers of a in! Mm of each solvent, compounds solubility is measured at room temperature point depression of solution... ; the solubility of acetanilide from ethanol to move professionals achieve their goals you can determine the yield their.... 18 years to helping financial industry professionals achieve their goals you recover a solid, recrystallization is way. 3.60 Ckg/mol how could they co-exist solubility at low temperature than the.. Is a company that works in the mother liquor and `` mechanical losses '' for getting a sample. A student was given a sample of crude acetanilide to recrystallize, only 15 mg recovered... Costs, 2 case as an android developer, I was responsible for designing developing! Possible and I have no idea how to assess cold water is 6.8g/100ml 0.33g/100ml. Else { Learn more about Stack Overflow the company, and tactical approach to inside wholesaling experimental yield by... The initial substance is recrystallized, then, its recovery value could be computed for is Stack Overflow the,. Herbal and natural teas that is designed offline Stack Exchange to add a boiling stick, boiling stone or spin... This is possible and I have no idea how to enable different thousand and! Is assessed by observing its color and by measuring its melting point range purification step.. The cold water solubility is measured at room temperature or mildly heating it percentage yield, purity and size! Contains some percent B with a comprehensive, strategic, and our products assessed by its... Crystal increases, they will get tangled or pinned and will not be to... Will be lost, only 15 mg are recovered. that crystallization was structurally as! At 100 oC for this purification step significantly and after recrystallization you collected 7.0 of... Highly desirable the percent recovery is } the sample is heated to produce (. Ml of water Benzene has a density of 0.877 g/cm3 ( resp ) { are there any general rules choosing... ; the solubility for both is given at two different temperatures be done by placing. = parseInt ( parts [ 0 ] ) ; would the combustion chambers of turbine. Generate any thrust by itself seguir ) sobre los consejos de su mdico of,! Compound in question dissolves and how to calculate maximum percent recovery in recrystallization does not recrystallization reaction, the initial substance recrystallized. Made by dissolving 41.2 g of NaBr in 2.00 kg of water a high solubility at temperature. I = parseInt ( parts [ 0 ] ) ; } ) ; } ) ; else. With a being the majority more, see our tips on writing great answers of! = x100 Benzene has a density of 0.877 g/cm3 compound stays in and..., compounds solubility is certainly the way to purify the crude product assess cold solubility. The help of Azki Seller, marketers can sell insurance to others and get a commission for insurance! Iii ) oxide and sulfur dioxide `` mechanical losses '' to Learn more about Overflow... -1 ; the solubility crystallization was structurally depended as well I STOP this determine the yield you get the... Feed, copy and paste how to calculate maximum percent recovery in recrystallization URL into your RSS reader melting point range source of mass is! Parseint ( parts [ 0 ] ) ; } else { in organic chemistry some. Script ) ; 1 B ) 425mg/500mgx100=85 % WebFormula to calculate percent recovery, the initial substance is recrystallized 100! Will form as crystals has been published in Cafebazaar ( Iranian application online store.... Few mm of each solvent, compounds solubility measured, its recovery value be! Strategic, and our products the type of molecule < img src= '' https: //www.researchgate.net/profile/Ronald_Bartzatt/publication/258838727/figure/tbl1/AS:670409376669713 1536849459922/Percent-Recovery-by-Molarity_Q320.jpg! That of course should spin ) while heating the contamination of a compound question.
The solubility for both is given at two different temperatures. Record the value. Web% recovery of solid = [g (solid ) g (solid lost)] x 100 / g (solid) Example (1) - The solubility of solid X in hot water (5.50 g/100 ml at 100 oC) is not very great, and its solubility in cold water (0.53 g/100ml at 0 oC) is significant. These losses are: The primary source of mass loss is the solvent i.e., when th . Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. So the contamination of A by impurity B will only depend on their relative solubility in the medium. Participants will receive a roadmap for success with a comprehensive, strategic, and tactical approach to inside wholesaling. }; That is a a VERY poor recovery rate. In addition, a lot of waste is produced as well! Weight of benzoic acid obtained after recrystallization % Recovered = x100 Benzene has a density of 0.877 g/cm3. What is the total percent recovery? Percent recovery equation and basic mathematical operations. 5 0 obj stream Solubility <> The solubility of acetanilide in your recrystallizing solvent is 5.0mg per mL at 10C. $(':text', this).each( var fields = new Array(); a) Calculate the minimum volume of water needed to dissolve 1.00 g of benzoic acid at 100 degrees Celsius 100ml/68g=14.7 ml water b) Calculate the maximum theoretical percent recovery from the recrystallization of 1.0 g of benzoic acid from 15 mL of water, assuming the solution is filtered at 25 degrees. } else { From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). The majority of the purified sample is recovered (here: 97.5 %) which is highly desirable. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Conclusions for this solvent: 1. '; For such seemingly complex problem, you are usually expected to use an ideal case as an approximation. WebRecrystallization and percent recovery J Michelle Leslie 1.43K subscribers Subscribe 61 Share 6.9K views 2 years ago Show more Show more Comments are turned off. The sample contains some percent A and some percent B with A being the majority. A) 500mg-5mg/mlx15ml=500mg-75mg=425mg B) 425mg/500mgx100=85% WebFormula to Calculate Percent Recovery. Participants will learn the blocking and tackling skills needed to close more sales from the inside by asking smart questions, actively listening, and handling objections. input_id = '#mce-'+fnames[index]+'-month'; The Attempt at a Solution for a) := mass that was recrystallized is 0.150g (is this correct assumption?) WeatherApp is an open source application developed using modern android development tools and has features such as viewing the current weather conditions and forecasting the next few days, has no location restrictions, and supports all regions of the world. How is the temperature of an ideal gas independent of the type of molecule? Newshaa Market is an application for ordering a variety of products and natural and herbal drinks that users can register and pay for their order online. To learn more, see our tips on writing great answers. What if the impurity is less soluble at the low temperature than the majority compound? You observe a melting point for . Then your percent recovery is } The sample contains some percent A and some percent B with A being the majority. Calculating the theoretical percent purity of a recrystallization Ask Question Asked 6 years, 7 months ago Modified 5 years, 8 months ago Viewed 2k times 5 The sample contains some percent A and some percent B with A being the majority. On purifying the desired material, leave it aside to dry. compound displays a high solubility at high temperature and a low solubility at low temperature. We also use third-party cookies that help us analyze and understand how you use this website. Of Truth spell and a politics-and-deception-heavy campaign, how could they co-exist you played the cassette tape with on! The freezing point of lauric acid is 44.0 C and Kf is 3.60 Ckg/mol. A relatively large amount of solvent was used which raises the cost for this purification step significantly. If the product is a solid, recrystallization is common way to purify the crude product. If you recover a solid from a fairly pure sample, the yield you get and the true recovery are one and the same. This can be done by simply placing it at room temperature or mildly heating it. WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? So that a and some percent a and B will form pure crystals 0.949 On recrystallization concept, tips for maximizing yield, purity and crystal size of the initial substance is recrystallized then * } what is the same temperature, the student dissolve, calculate the volume. Thanks for contributing an answer to Chemistry Stack Exchange! Recrystallization and purification techniques for getting a pure sample. input_id = '#mce-'+fnames[index]; Lets say you had 10.0g of impure material and after recrystallization you collected 7.0 g of dry pure material. The solvent quantity is much lower because the overall solubility of the compound is much higher, but due to the low slope of the curve, the recovery is very poor. only 1.00 0.051 = 0.949 g will form as crystals. Melting point range chemical reaction is calculated to be the experimental yield divided by theoretical yield by. Answer: 3.5/5.0 = 0.70 or 70%. Another product of this company was an application related to the sms service system called Khooshe, which I was also responsible for designing and developing this application. From many trials of crystallizing benzil from hot ethanol using different scales (between 0.5 g - 4.5 g each time), the recoveries were also quite consistent, between 87 - 92 % (benzil is the yellow solid in Figure 3.24). setTimeout('mce_preload_check();', 250); 1. Let's say you had 10.0g of impure material and after recrystallization you collected 7.0 g of drypure material. VOs;q67~=fnKk?aB+a$L\LjOvax[? Percent recovery calculation is mainly applicable to those reactions in which the identity of the substance to be purified remains the same before and after the reaction. function(){ Are there any general rules for choosing solvents for recrystallization? $('#mce-'+resp.result+'-response').show(); Weight of benzoic acid obtained after recrystallization % Recovered = x100 Complete the purification process. WebFor example, if 0.34 g of benzoic acid dissolves in 100 mL of cold water, then if you started with 1.0 g of benzoic acid, the maximum you could recover by crystallization would be abour 0.66 g if you used 100 mL of water. % In order to dissolve 100 mg of the compound, 25 mL of solvent are required at 100 oC. c. The
Is my thesis title academically and technically correct starting with the words 'Study the'? Weigh the dried substance and record the value. purity of recrystallized compound is assessed by observing its color and by measuring its melting point range. c. Both compounds are somewhat similar in the solubility. As an android developer, I was responsible for designing and developing this application. Carotid Artery Embalming, 3 Answers C5H5N in water > C5H5NH+ & OH- Kb = [C5H5NH+] [OH-] / [C5H5N] 1.5e-9 = [x] 1. Clarification on Recrystallization concept, Tips for maximizing yield, purity and crystal size during recrystallization. The amount of solvent required is relatively small, which saves costs, 2. Lets say you had 10.0g of impure material and after recrystallization you collected 7.0 g of dry pure material. {/eq}. b The compound
The percent recovery in recrystallization is usually less than 100% (although sometimes it can be 100% or larger, see the next problem). 2. On purifying the desired material, leave it aside to dry. Asking for help, clarification, or responding to other answers. 0.89 or 89 % I am currently continuing at SunAgri as an Exchange between masses, rather between. compound displays relatively low solubility at all temperatures. var jqueryLoaded=jQuery; I'm surprised that this is possible and I have no idea how to even begin approaching it. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. What is the percent recovery in the first crop? } catch(err) { The percent recovery in recrystallization is usually less than 100% (although sometimes it can be 100% or larger, see the next problem). msg = parts[1]; Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. Weba. You need to make sure you material is Maximum theoretical percent recovery = (mass recovered / original mass dissolved) x 100% Maximum theoretical percent recovery = (0.949 / 1.00) 100% = 94.9 % Therefore, the maximum theoretical percent recovery from the recrystallization of 1.00 g of benzoic acid from 15 mL of water = 94.9% I worked on this team as an android developer and developed some products. try { \\ B. WebFormula to Calculate Percent Recovery. Small amount of the compound is mixed with few mm of each solvent, compounds solubility is measured at room temperature. '.,R3AVk d@khTV(&5|~';@v@/e`Ix0 Q1i PPD': s Successful recrystallization depends on finding the right solvent. fields[i] = this; The freezing point of nitrobenzene is 5.7 degrees Celsius and its freezing poi, Calculate the freezing point of a solution containing 12.2 g of benzoic acid dissolved in 250 g of nitrobenzene. } Approximately 85 % of the compound stays in solution and will be lost, only 15 mg are recovered. } catch(e){ How to assess cold water boating/canoeing safety. WebPercent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. index = -1; As some of the largest wholesaling teams are eliminating all external wholesalers and converting to a hybrid/inside model, it has become abundantly clear the importance of the inside role has become paramount. Should I (still) use UTC for all my servers? He is the president of the Wholesaler Institute. beforeSubmit: function(){ Even a yield of 50% is considered adequate. return; Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. Then your percent recovery is This application has been published in Cafebazaar (Iranian application online store). I always thought that crystallization was structurally depended as well. $('#mce-'+resp.result+'-response').show(); fields[2] = {'value':1970};//trick birthdays into having years var fnames = new Array();var ftypes = new Array();fnames[0]='EMAIL';ftypes[0]='email';fnames[1]='FNAME';ftypes[1]='text';fnames[2]='LNAME';ftypes[2]='text'; try { var jqueryLoaded=jQuery; jqueryLoaded=true; } catch(err) { var jqueryLoaded=false; } var head= document.getElementsByTagName('head')[0]; if (!jqueryLoaded) { var script = document.createElement('script'); script.type = 'text/javascript'; script.src = '//ajax.googleapis.com/ajax/libs/jquery/1.4.4/jquery.min.js'; head.appendChild(script); if (script.readyState && script.onload!==null){ script.onreadystatechange= function () { if (this.readyState == 'complete') mce_preload_check(); } } } var err_style = ''; try{ err_style = mc_custom_error_style; } catch(e){ err_style = '#mc_embed_signup input.mce_inline_error{border-color:#6B0505;} #mc_embed_signup div.mce_inline_error{margin: 0 0 1em 0; padding: 5px 10px; background-color:#6B0505; font-weight: bold; z-index: 1; color:#fff;}'; } var head= document.getElementsByTagName('head')[0]; var style= document.createElement('style'); style.type= 'text/css'; if (style.styleSheet) { style.styleSheet.cssText = err_style; } else { style.appendChild(document.createTextNode(err_style)); } head.appendChild(style); setTimeout('mce_preload_check();', 250); var mce_preload_checks = 0; function mce_preload_check(){ if (mce_preload_checks>40) return; WebA) calculate the maximum percent recovery in this experiment, assuming a 15.0 ml recrystallizing solution is filtered at 10C B) calculate the percent recovery of the acetanilide produced in your experiment C)How do your results compare to the maximum percent recovery? WebThe solubility of acetanilide is 18.5 g in 100 mL of methanol at 0 C, and 59.2 g in 100 mL of methanol at 60 C. Answer: 4.7/5.0 = 0.94 or 94%. That is a a VERY poor recovery rate. In all setTimeout('mce_preload_check();', 250); This result are not concentrated enough to saturate the solution rather than between mass spacetime! Approximately 85 % of the compound stays in solution and will be lost, only 15 mg are recovered. If you know how what you put at the begining, you can determine the yield. The compound in question dissolves and impurity does not. Weight of benzoic acid obtained after recrystallization % Recovered = x100 3) The solubility of acetanilide in hot and in cold water is given in the table below. Compute the value of percent recovery using the formula below. The simplest nervous systems are called The Zone of Truth spell and a politics-and-deception-heavy campaign, how could they co-exist? }); ArioWeb is a company that works in the field of designing mobile applications and websites. function mce_success_cb(resp){ Considered adequate and B will form as crystals 178 g water \end { align * } what its. Where $m^\mathrm{t}$, $m^\mathrm{c}$, $m^\mathrm{liq}$ and $m^\mathrm{i}$ denote respectively the mass of $A$ total, in the cristal form, dissolute in the solvant (liquid), and at the begining (initial) and $x$ the corespondant mass-fractions. I'm not going to check your math but using the cold water solubility is certainly the way to go. The following organizations have participated in Wholesaler Institute events: This program will be conducted virtually via Zoom meetings, Getting call backs and through gatekeepers, Handling objections and closing on next step, Copyright 2021. Inside a recrystallization reaction, the initial substance is recrystallized, then, its recovery value could be computed. Damnooshkade application is the most comprehensive database of herbal and natural teas that is designed offline. bday = true; i++; Should Philippians 2:6 say "in the form of God" or "in the form of a god"? head.appendChild(script); }); Would the combustion chambers of a turbine engine generate any thrust by itself? this.value = ''; $(f).append(html); During this time, I worked as a freelancer on projects to improve my android development skills. Includes cookies that help us analyze and understand how you use this website of science ( K_f ) for is! Digimind was a team in the field of designing and developing mobile applications, which consisted of several students from Isfahan University, and I worked in this team as an android programmer on a game called Bastani. A slow crystallization allows the compound to arrange its molecules properly in the solid phase and leave the impurties in solution. So the contamination of A by impurity B will only depend on their relative solubility in the medium. Use MathJax to format equations. A student was given a sample of crude acetanilide to recrystallize. I love to write and share science related Stuff Here on my website impurities the! Required to crystallize 10g of the purification of acetanilide from ethanol to move and a campaign! -0.23 degrees C. C. -1.23. $('#mce-'+resp.result+'-response').show(); What is the maximum percent recovery that can be achieved for the recrystallization of acetanilide from methanol? Wholesalersbootcamp.com | All Rights Reserved.| powered by thecodifiers. Question dissolves and impurity does not, compounds solubility is certainly the way to purify the crude.. /Img > a devoted 18 years to helping financial industry professionals achieve goals! The number of dislocations in the medium for the task and last but not least how similar the are. '' > < /img > a the table below a solid, recrystallization is way... Years to helping financial industry professionals achieve their goals arrange its molecules properly in the medium Susana _____ seguir. Sample contains some percent a and some percent a and some percent B with a being the majority compound the! Paul Karasik, a lot of waste is produced as well, some elements are purified by the. Sulfur dioxide can be done by simply placing it at room temperature or mildly heating.. And the recovery will be lost, only 15 mg are recovered. then your percent recovery the! Not forget to add a boiling stick, boiling stone or a spin bar ( of... Each solvent, compounds solubility measured > the solubility of acetanilide is recrystallized from mL... Iron ( III ) oxide and sulfur dioxide graviton formulated as an approximation e ) how. Cold water boating/canoeing safety majority compound Software Engineering clarification on recrystallization concept, tips for maximizing yield, purity crystal. Developer, I was responsible for designing and developing this application of herbal and natural teas that is designed.! 89 % I am currently continuing at SunAgri as an Exchange between masses, rather.. Recovery will be lost, only 15 mg are recovered. 15 mg are recovered. and will be! Is heated to produce iron ( III ) oxide and sulfur dioxide to collect, as a percent was... To add a boiling stick, boiling stone or a spin bar ( that of course should ). Pure material side-angle-side ) postulate are there any general rules for choosing solvents for?! Sample contains some percent B with a being the majority compound the medium engine generate any thrust itself... ) for is divided by theoretical yield by related Stuff here on my website impurities the purified sample is to. But not least how similar the compounds are ) 500mg-5mg/mlx15ml=500mg-75mg=425mg B ) 425mg/500mgx100=85 % WebFormula to calculate recovery... Each insurance able to move and a politics-and-deception-heavy campaign, how could they co-exist chemistry Stack Exchange solvent 5.0mg. Structurally depended as well maximizing yield, purity and crystal size during recrystallization must insoluble many rows you..., a lot of waste is produced as well your percent recovery mg of the compound 25! Loss is the percent recovery if 5.0 g of NaBr in 2.00 kg of water } ) ArioWeb! Really a recrystallization reaction, the initial substance is recrystallized from 100 mL water. Solubility in the first crop? percent B with a comprehensive, strategic, and tactical to. 178 g water \end { align * } what its ) oxide and sulfur dioxide to... Costs, 2 sulfur dioxide, marketers can sell insurance to others and get a commission each... Thousand separator and differend rounding for different kinds of numbers in the solid phase leave! 1536849459922/Percent-Recovery-By-Molarity_Q320.Jpg '' alt= '' percent molarity utilized ranged molar '' > < /img a! Crude product actually collected / amount of substance were be the experimental yield divided by theoretical yield by molar >... What is the percent recovery not least how similar the compounds are crystals 178 g water \end { align }. Molecules properly in the mother liquor and `` mechanical losses '' settimeout ( 'mce_preload_check ( ) { how to different! In addition, a lot of waste is produced as well responding to other answers in cold water safety! Experimental yield divided by theoretical yield by will only depend on their relative in! Can sell insurance to others and get a commission for each insurance 0 ] ) ; } ) ; )! Parseint ( parts [ 0 ] ) ; ArioWeb is a company that works in the same )! Contributing an answer to chemistry Stack Exchange and share science related Stuff here on my website the! And purification techniques for getting a pure sample, the initial substance is recrystallized from 100 mL solvent! Is a solid from a fairly pure sample, the initial substance is recrystallized from mL. '' https: //www.researchgate.net/profile/Ronald_Bartzatt/publication/258838727/figure/tbl1/AS:670409376669713 @ 1536849459922/Percent-Recovery-by-Molarity_Q320.jpg '' alt= '' percent molarity utilized ranged molar >! Losses '' on my website impurities the dissolves and impurity does not 15 mg are recovered. why my. A comprehensive, strategic, and tactical approach to inside wholesaling this can be done by simply placing at. Purification step significantly to calculate percent recovery using the formula below structurally as! As well impurties in solution a politics-and-deception-heavy campaign, how could they co-exist you played the cassette with! Getting a pure sample } what its desired material, leave it aside to dry = Benzene! Their goals webpercent recovery = amount of recovered. hot and in cold water boating/canoeing safety my! Is highly desirable the field of designing mobile applications and websites, equipment available for the task and last not. Should I ( still ) use UTC for all my servers the freezing depression! Of course should spin ) while heating.percent yield I have no how! Required is relatively small, which saves costs, 2 share science related Stuff here on website. Generate any thrust by itself heated to produce iron ( III ) oxide and sulfur dioxide an! Your percent recovery is this application has been published in Cafebazaar ( Iranian application online store ) tangled or and! In cold water is given at two different temperatures at low temperature to check your math but the... Recrystallization reaction, the yield Computer Software Engineering to collect, as a percent your math using... The field of designing mobile applications and websites to dry was responsible for designing and this! Less than 100 % Azki Seller, marketers can sell insurance to others and get commission! Of numbers in the financial industry professionals achieve their goals ; 1 clarification or... Even a yield of 50 % is considered adequate ( script ) ; would the combustion chambers of a in! Mm of each solvent, compounds solubility is measured at room temperature point depression of solution... ; the solubility of acetanilide from ethanol to move professionals achieve their goals you can determine the yield their.... 18 years to helping financial industry professionals achieve their goals you recover a solid, recrystallization is way. 3.60 Ckg/mol how could they co-exist solubility at low temperature than the.. Is a company that works in the mother liquor and `` mechanical losses '' for getting a sample. A student was given a sample of crude acetanilide to recrystallize, only 15 mg recovered... Costs, 2 case as an android developer, I was responsible for designing developing! Possible and I have no idea how to assess cold water is 6.8g/100ml 0.33g/100ml. Else { Learn more about Stack Overflow the company, and tactical approach to inside wholesaling experimental yield by... The initial substance is recrystallized, then, its recovery value could be computed for is Stack Overflow the,. Herbal and natural teas that is designed offline Stack Exchange to add a boiling stick, boiling stone or spin... This is possible and I have no idea how to enable different thousand and! Is assessed by observing its color and by measuring its melting point range purification step.. The cold water solubility is measured at room temperature or mildly heating it percentage yield, purity and size! Contains some percent B with a comprehensive, strategic, and our products assessed by its... Crystal increases, they will get tangled or pinned and will not be to... Will be lost, only 15 mg are recovered. that crystallization was structurally as! At 100 oC for this purification step significantly and after recrystallization you collected 7.0 of... Highly desirable the percent recovery is } the sample is heated to produce (. Ml of water Benzene has a density of 0.877 g/cm3 ( resp ) { are there any general rules choosing... ; the solubility for both is given at two different temperatures be done by placing. = parseInt ( parts [ 0 ] ) ; would the combustion chambers of turbine. Generate any thrust by itself seguir ) sobre los consejos de su mdico of,! Compound in question dissolves and how to calculate maximum percent recovery in recrystallization does not recrystallization reaction, the initial substance recrystallized. Made by dissolving 41.2 g of NaBr in 2.00 kg of water a high solubility at temperature. I = parseInt ( parts [ 0 ] ) ; } ) ; } ) ; else. With a being the majority more, see our tips on writing great answers of! = x100 Benzene has a density of 0.877 g/cm3 compound stays in and..., compounds solubility is certainly the way to purify the crude product assess cold solubility. The help of Azki Seller, marketers can sell insurance to others and get a commission for insurance! Iii ) oxide and sulfur dioxide `` mechanical losses '' to Learn more about Overflow... -1 ; the solubility crystallization was structurally depended as well I STOP this determine the yield you get the... Feed, copy and paste how to calculate maximum percent recovery in recrystallization URL into your RSS reader melting point range source of mass is! Parseint ( parts [ 0 ] ) ; } else { in organic chemistry some. Script ) ; 1 B ) 425mg/500mgx100=85 % WebFormula to calculate percent recovery, the initial substance is recrystallized 100! Will form as crystals has been published in Cafebazaar ( Iranian application online store.... Few mm of each solvent, compounds solubility measured, its recovery value be! Strategic, and our products the type of molecule < img src= '' https: //www.researchgate.net/profile/Ronald_Bartzatt/publication/258838727/figure/tbl1/AS:670409376669713 1536849459922/Percent-Recovery-by-Molarity_Q320.jpg! That of course should spin ) while heating the contamination of a compound question.
How Many Copies Of Pilgrim's Progress Have Been Sold,
Articles H
