methanol production from syngas mass balance
J. Thesis, Chemical and Petroleum Engineering, University of Kansas, Sara M, Rouissi T, Brar SK, Blais JF (2016) Propylene glycol: an industrially important C3 platform chemical. Available: https://www.sasol.com/sites/sasol/files/content/files/Sasol%2050%20year%20Brochure_1039069422306_0_0_1.pdf. Accessed 15 July2022, Galera S, Ortiz FG (2015) Techno-economic assessment of hydrogen and power production from supercritical water reforming of glycerol. CRC Press, Boca Raton, Florida, Giuliano A, Freda C, Catizzone E (2020) Techno-economic assessment of bio-syngas production for methanol synthesis: A focus on the watergas shift and carbon capture sections. Common Energy 97, 6784 (2013), Narobe, M., Golob, J., Klinar, D., Franceti, V., Likozar, B.: Co-gasification of biomass and plastics: pyrolysis kinetics studies, experiments on 100kW dual fluidized bed pilot plant and development of thermodynamic equilibrium model and balances. At the adopted temperature (250 ) and also at the widely used temperature (265 ) in methanol production, the effect of pressure on syngas component conversion and methanol synthesis was investigated. Biomass-derived energy sources are thought to be more environmentally friendly, particularly in the transportation sector [8]. Fluidized bed technologies for near-zero emission combustion and gasification, 1st edn., pp. WebWith the considerations of the complex kinetic mechanisms of the methanol steam reforming using Cu/ZnO/Al2O3 catalyst, in this paper a multiphysics coupling model that integrates the mass, momentum and energy conservation governing equations is proposed to investigate the thermophysical performances of the mid-and-low temperature solar receiver/reactor for Concentrating solar power technology, pp. WebProduction [ edit] Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. In addition, Lcking [50] and Leonzio [53] both observed from their experiments that all the reactants react to generate methanol when \({S}_{N}\) is equal to 2, this was also the case in this study where all the syngas components participated in the methanol synthesis process. Int. Moreover, the export of biochar as a co-product resulted in a 35% decrease in potential environmental impact. The yield from the reactors as well as overall yield of methanol for each purity of crude glycerol were computed as shown in Table 5. Process Chemistry  while those of the costing and economics can be found in Tables S14-S19 in the supporting document. The datasets generated throughout the completion of the current study can be obtained from the corresponding author on reasonable request. A conceptual design for the production of synthesis gas, suitable for methanol production, is presented. Fuel 318:123650, Maneewuthiworasakul M, Patthaveekongka W (2019) Direct methanol synthesis from glycerol over modified basic metal oxide catalysts," Doctoral Dissertation, Silpakorn University, Roslan NA, Abidin SZ, Ideris A, Vo DVN (2020) A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Food Ind. Effect of SGR on the syngas composition at (a) STR=625 (b) STR=650 . Springer Nature, Switzerland, pp 101125, Carr AG, Shi X, Domene C, Leung AK, Green WH (2016) Methanol formation from the treatment of glycerol in supercritical water and with ethylsulfide. A similar result was reported by Adji et al. According to Eq. Examples of Technology and Plant: The purpose of this region is to reduce the moisture content of the feedstock. Catalysts 9(1):15, Plcido J, Capareda S (2016) Conversion of residues and by-products from the biodiesel industry into value-added products. WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm total sulfur, including hydrogen sulfide (H2S) and carbonyl sulfide (COS), and P.C. As an illustration, crude glycerol can be used to create hydrogen and syngas, which are frequently used as primary feedstocks for the synthesis of valuable compounds like methanol [18, 28]. Being highly endothermic, glycerol STR requires high temperatures, moderate pressures, and partially high SGR in order to achieve high conversion. This process results in a considerable hydrogen surplus, as can be seen. The effect of STR temperature is illustrated in Fig. Methanol is a key ingredient in the production of biodiesel and other high-value compounds [21]. Ed il tuo sito o per includere alcuni crediti, 10 ] stesso il! Investigations were done into how varying these parameters affected the methanol synthesis and the conversion of the main syngas components (i.e., H2, CO, CO2, and CH4). Therefore, at the reactors designated temperature, significant CO conversion is required. L.A. ; Bhattacharyya, D. Choi, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential alcuni.! According to the methanation reaction in Equation (6) the mole fraction of CH4 in syngas decreases and that of H2 increases with the increase in temperature. [18], some of the purified H2 stream can be used to run the furnace or routed to a proton-exchange membrane fuel cell for power production. Reforming glycerol to hydrogen, syngas, acrolein, propylene glycol, methanol, and other valuable compounds is an example of a possible conversion process [16, 17]. [36], who both reported that the exothermic nature of the methanation reaction makes the CH4 production insignificant at temperatures above 600 . The authors would like to thank the University of Johannesburg for its support. Major technology providers include: From 2011 to 2014, nearly 11 GWth syngas capacity for methanol production started up at several new coal or lignite gasification-based plants in China. And production network consisting of LNG, GTL, and the final methanol catalytic synthesis the production costs process! 8 are independent processes that are adequate to determine the ratio of reactants to products at equilibrium [3]. 395420. Am. These costs were updated to present value using the widely used chemical engineering plant cost index (CEPCI) [71]. As the base case, Purity A obtained from Tamoinas et al. As mentioned earlier, the standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. Due to the large amount of crude glycerol produced as a by-product by the biodiesel industry, alternative technologies for converting glycerol to value-added fuels such as syngas have been proposed. RStoic converts a part of feed to form water which requires the extent of reaction known as: The yield of gaseous water is determined by the water content in the proximate analysis of particular feedstock. : Uncatalyzed and wall-catalyzed forward watergas shift reaction kinetics. [62], the molar proportion of CH4 drops as temperature increases. Chem. The temperature of the process affects the stoichiometric coefficients. 10. Low amounts of these unreacted components were recycled back in the equilibrium reactor when compared to stoichiometry reactor observed by Ortiz et al. [18] with 20% and 3% CO and CO2 conversions per pass respectively. Eng. 23(10), 14771491 (1999), Cabezas, H., Bare, J.C., Mallick, S.K. The supplementary data on the process simulation can be found in Tables S1-S 13 and Fig. Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this website. The stream carrying the crude methanol (S-16) was introduced into the purification stage, where it is removed from dissolved gas and water. Thus, in order to prevent the creation of CH4 and coke, the STR of crude glycerol must be carried out at high temperatures. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). N. Seedat: Conceptualization, Supervision, Investigation, Writing reviewing and editing. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. Energy 86(9), 22932305 (2012), Diago, M., Iniesta, A.C., Delclos, T., Soum-Glaude, A., Shamim, T., Calve, N.: Characterization of desert sand as a sensible thermal energy storage medium. 2, the raw feed streams (i.e., water and crude glycerol) were introduced at room temperature (25 ), and a pressure of 1bar into the system. Dissolved gases were discovered to be present in S-16, hence, the two-column distillation was employed in this study. Korean J Chem Eng 27(6):17601767, Adji BS, Muharam Y, Kartohardjono S (2018) Simulation of methanol synthesis in packed bed reactor for utilization of CO2 from acid gas removal unit. The Aspen Plus simulation software was used to simulate the conversion process from syngas into Conversion of carbon sources to methanol c). A promising and environmentally friendly source for renewable methanol is glycerol [22]. MATH In 2021, worldwide methanol production reached 107 million metric tons. Master of Science Thesis, School of Chemical and Bio Engineering, Addis Ababa Institute of Technology (AAiT), Addis Ababa University, Ethiopia, Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass. Laitinen [61] asserts that the production of methanol takes place between 220280 . Continuing to use this website means you agree to our use of cookies. The physicochemical properties of fuels were determined by using the Peng-Robinson equation of state as proposed in the work of Mousavi Ehteshami & Chan [55]. Yakan Nwai, C., Patel, B. Techno-Economic Study and Environmental Analysis for the Production of Bio-methanol Using a Solar-Aided Dual-bed Gasifier. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Comput. The CGtM process configuration was divided into four key sections which include the syngas production (from STR of crude glycerol) section, the gas treatment section (using pressured swing adsorption (PSA) system), and the methanol synthesis section, and finally, the crude methanol purification section. In: Ashock Pandey, J.C.P.C.H.C.L. Bioref. Environ Progress Sustain Energy Fuels 35(6):17651771, Lin Y-C (2013) Catalytic valorization of glycerol to hydrogen and syngas. From 2008 through 2035, global energy-linked CO2 emission is predicted to increase by 43% if petroleum-based resources are used [4]. Ind Eng Chem Res 49(13):61506163, Bozzano G, Manenti F (2016) Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Energy Chem. AIP Conf. The methanol synthesis section is where methanol is produced after the compressed syngas has been transferred there. The least sensitive variables are the working capital, loan interest rate and the discount rate. When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3]. In addition, Purities A and B have the highest methanol concentration in the final products stream as shown in Table 5 with 98% and 99% respectively. Sustainability 14(4):2189, Jenkins S (2022) Chemical Engineering Plant Cost Index. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. [36] reported similar findings when examining how SGR affected syngas composition, noting a decline in the syngas composition. 3. Chemical Engineering questions and answers. [40], the presence of a catalyst allows for the reduction of SCWR operating expenses as well as process conditions including temperature and energy requirements. Adewale George Adeniyi & Joshua O. Ighalo, Zulfiqar Ali Bhatti, Sania Bhatti, Ghulamullah Maitlo, Amira Nemmour, Chaouki Ghenai & Abrar Inayat, Tala Alsamad, Manar Almazrouei, Isam Janajreh, Ammaru Ismaila, Xueli Chen, Xiaolei Fan, Mohammad Tazli Azizan, Aqsha Aqsha, Farooq Sher, Biomass Conversion and Biorefinery Eng. It can be shown that the PSA system has improved SN value to 2 using the stream S-8 syngas component (see Table 3). By employing four main processes, the syngas could 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. Process flowsheet for methanol production from crude glycerol. In addition, to prevent the build-up of inert gases, mostly methane, 1% of the recompressed gas stream was purged off. In principle the process is conducted in two steps. The material balance around the steam reforming (STR) and pressure swing adsorption (PSA) section is presented in Table 3. Biomass Bioenerg. Depending on the catalyst supplier, the synthesis reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F. J. SASOL, SASOL, 50 years of innovation, 2000. Int J Hydrogen Energy 47(3):14611480, Martn M, Grossmann IE (2017) Towards zero CO2 emissions in the production of methanol from switchgrass. Nonetheless, the effect of varying the synthesis temperature and pressure was also investigated in the subsequent section.
while those of the costing and economics can be found in Tables S14-S19 in the supporting document. The datasets generated throughout the completion of the current study can be obtained from the corresponding author on reasonable request. A conceptual design for the production of synthesis gas, suitable for methanol production, is presented. Fuel 318:123650, Maneewuthiworasakul M, Patthaveekongka W (2019) Direct methanol synthesis from glycerol over modified basic metal oxide catalysts," Doctoral Dissertation, Silpakorn University, Roslan NA, Abidin SZ, Ideris A, Vo DVN (2020) A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Food Ind. Effect of SGR on the syngas composition at (a) STR=625 (b) STR=650 . Springer Nature, Switzerland, pp 101125, Carr AG, Shi X, Domene C, Leung AK, Green WH (2016) Methanol formation from the treatment of glycerol in supercritical water and with ethylsulfide. A similar result was reported by Adji et al. According to Eq. Examples of Technology and Plant: The purpose of this region is to reduce the moisture content of the feedstock. Catalysts 9(1):15, Plcido J, Capareda S (2016) Conversion of residues and by-products from the biodiesel industry into value-added products. WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm total sulfur, including hydrogen sulfide (H2S) and carbonyl sulfide (COS), and P.C. As an illustration, crude glycerol can be used to create hydrogen and syngas, which are frequently used as primary feedstocks for the synthesis of valuable compounds like methanol [18, 28]. Being highly endothermic, glycerol STR requires high temperatures, moderate pressures, and partially high SGR in order to achieve high conversion. This process results in a considerable hydrogen surplus, as can be seen. The effect of STR temperature is illustrated in Fig. Methanol is a key ingredient in the production of biodiesel and other high-value compounds [21]. Ed il tuo sito o per includere alcuni crediti, 10 ] stesso il! Investigations were done into how varying these parameters affected the methanol synthesis and the conversion of the main syngas components (i.e., H2, CO, CO2, and CH4). Therefore, at the reactors designated temperature, significant CO conversion is required. L.A. ; Bhattacharyya, D. Choi, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential alcuni.! According to the methanation reaction in Equation (6) the mole fraction of CH4 in syngas decreases and that of H2 increases with the increase in temperature. [18], some of the purified H2 stream can be used to run the furnace or routed to a proton-exchange membrane fuel cell for power production. Reforming glycerol to hydrogen, syngas, acrolein, propylene glycol, methanol, and other valuable compounds is an example of a possible conversion process [16, 17]. [36], who both reported that the exothermic nature of the methanation reaction makes the CH4 production insignificant at temperatures above 600 . The authors would like to thank the University of Johannesburg for its support. Major technology providers include: From 2011 to 2014, nearly 11 GWth syngas capacity for methanol production started up at several new coal or lignite gasification-based plants in China. And production network consisting of LNG, GTL, and the final methanol catalytic synthesis the production costs process! 8 are independent processes that are adequate to determine the ratio of reactants to products at equilibrium [3]. 395420. Am. These costs were updated to present value using the widely used chemical engineering plant cost index (CEPCI) [71]. As the base case, Purity A obtained from Tamoinas et al. As mentioned earlier, the standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. Due to the large amount of crude glycerol produced as a by-product by the biodiesel industry, alternative technologies for converting glycerol to value-added fuels such as syngas have been proposed. RStoic converts a part of feed to form water which requires the extent of reaction known as: The yield of gaseous water is determined by the water content in the proximate analysis of particular feedstock. : Uncatalyzed and wall-catalyzed forward watergas shift reaction kinetics. [62], the molar proportion of CH4 drops as temperature increases. Chem. The temperature of the process affects the stoichiometric coefficients. 10. Low amounts of these unreacted components were recycled back in the equilibrium reactor when compared to stoichiometry reactor observed by Ortiz et al. [18] with 20% and 3% CO and CO2 conversions per pass respectively. Eng. 23(10), 14771491 (1999), Cabezas, H., Bare, J.C., Mallick, S.K. The supplementary data on the process simulation can be found in Tables S1-S 13 and Fig. Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this website. The stream carrying the crude methanol (S-16) was introduced into the purification stage, where it is removed from dissolved gas and water. Thus, in order to prevent the creation of CH4 and coke, the STR of crude glycerol must be carried out at high temperatures. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). N. Seedat: Conceptualization, Supervision, Investigation, Writing reviewing and editing. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. Energy 86(9), 22932305 (2012), Diago, M., Iniesta, A.C., Delclos, T., Soum-Glaude, A., Shamim, T., Calve, N.: Characterization of desert sand as a sensible thermal energy storage medium. 2, the raw feed streams (i.e., water and crude glycerol) were introduced at room temperature (25 ), and a pressure of 1bar into the system. Dissolved gases were discovered to be present in S-16, hence, the two-column distillation was employed in this study. Korean J Chem Eng 27(6):17601767, Adji BS, Muharam Y, Kartohardjono S (2018) Simulation of methanol synthesis in packed bed reactor for utilization of CO2 from acid gas removal unit. The Aspen Plus simulation software was used to simulate the conversion process from syngas into Conversion of carbon sources to methanol c). A promising and environmentally friendly source for renewable methanol is glycerol [22]. MATH In 2021, worldwide methanol production reached 107 million metric tons. Master of Science Thesis, School of Chemical and Bio Engineering, Addis Ababa Institute of Technology (AAiT), Addis Ababa University, Ethiopia, Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass. Laitinen [61] asserts that the production of methanol takes place between 220280 . Continuing to use this website means you agree to our use of cookies. The physicochemical properties of fuels were determined by using the Peng-Robinson equation of state as proposed in the work of Mousavi Ehteshami & Chan [55]. Yakan Nwai, C., Patel, B. Techno-Economic Study and Environmental Analysis for the Production of Bio-methanol Using a Solar-Aided Dual-bed Gasifier. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Comput. The CGtM process configuration was divided into four key sections which include the syngas production (from STR of crude glycerol) section, the gas treatment section (using pressured swing adsorption (PSA) system), and the methanol synthesis section, and finally, the crude methanol purification section. In: Ashock Pandey, J.C.P.C.H.C.L. Bioref. Environ Progress Sustain Energy Fuels 35(6):17651771, Lin Y-C (2013) Catalytic valorization of glycerol to hydrogen and syngas. From 2008 through 2035, global energy-linked CO2 emission is predicted to increase by 43% if petroleum-based resources are used [4]. Ind Eng Chem Res 49(13):61506163, Bozzano G, Manenti F (2016) Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Energy Chem. AIP Conf. The methanol synthesis section is where methanol is produced after the compressed syngas has been transferred there. The least sensitive variables are the working capital, loan interest rate and the discount rate. When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3]. In addition, Purities A and B have the highest methanol concentration in the final products stream as shown in Table 5 with 98% and 99% respectively. Sustainability 14(4):2189, Jenkins S (2022) Chemical Engineering Plant Cost Index. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. [36] reported similar findings when examining how SGR affected syngas composition, noting a decline in the syngas composition. 3. Chemical Engineering questions and answers. [40], the presence of a catalyst allows for the reduction of SCWR operating expenses as well as process conditions including temperature and energy requirements. Adewale George Adeniyi & Joshua O. Ighalo, Zulfiqar Ali Bhatti, Sania Bhatti, Ghulamullah Maitlo, Amira Nemmour, Chaouki Ghenai & Abrar Inayat, Tala Alsamad, Manar Almazrouei, Isam Janajreh, Ammaru Ismaila, Xueli Chen, Xiaolei Fan, Mohammad Tazli Azizan, Aqsha Aqsha, Farooq Sher, Biomass Conversion and Biorefinery Eng. It can be shown that the PSA system has improved SN value to 2 using the stream S-8 syngas component (see Table 3). By employing four main processes, the syngas could 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. Process flowsheet for methanol production from crude glycerol. In addition, to prevent the build-up of inert gases, mostly methane, 1% of the recompressed gas stream was purged off. In principle the process is conducted in two steps. The material balance around the steam reforming (STR) and pressure swing adsorption (PSA) section is presented in Table 3. Biomass Bioenerg. Depending on the catalyst supplier, the synthesis reaction is normally carried out at about 600 to 1,700 psig and 400 to 600F. J. SASOL, SASOL, 50 years of innovation, 2000. Int J Hydrogen Energy 47(3):14611480, Martn M, Grossmann IE (2017) Towards zero CO2 emissions in the production of methanol from switchgrass. Nonetheless, the effect of varying the synthesis temperature and pressure was also investigated in the subsequent section.  As mentioned earlier, the KPIs considered in this study include the ROI, NPV, DPBP, and NPC. The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. N. Seedat. Fuel 144:307316. [Online]. This section examines the effect of changing the temperature of the methanol synthesis reaction while maintaining the base pressure of 80bar and also a commonly used pressure of 75bar for effective comparison. The data for the analysis are available in the supporting documents (see costing and economics supplementary information). H 2 + O 2 H 2O H -57.8 kcal/mole zH 2 is an energy vector, is converted to water which has minimal environmental impact. However, high SGR help the methane reforming and watergas-shift reactions progress toward the formation of H2 [45]. Ph. In order to increase the overall conversion of CO to methanol, the gas was compressed further with a multi-stage compressor and then recycled. Tax calculation will be finalised during checkout. It combines the partial oxidation (POX) process with the STR process [28]. Webmethanol [1] [2]. Before the synthesis of biodiesel is technically and economically practical, there are some difficulties associated with its use that must be overcome first [12]. The APC was estimated to be around $38.9 million. /> [153] found that active copper species is predominantly present as Cu0 over Cu/ZrO2 based on X-ray diffraction measurements. Christian Yakan Nwai. Of with have studied the increase in methanol conversion and the decrease the! However, at temperatures above 280 , the catalyst would be prone to sintering and fusion, which would permanently damage the catalyst [18, 68].
As mentioned earlier, the KPIs considered in this study include the ROI, NPV, DPBP, and NPC. The production of syngas from carbonaceous feedstock is typically performed using either direct/autothermal or indirect/allothermal gasification. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. N. Seedat. Fuel 144:307316. [Online]. This section examines the effect of changing the temperature of the methanol synthesis reaction while maintaining the base pressure of 80bar and also a commonly used pressure of 75bar for effective comparison. The data for the analysis are available in the supporting documents (see costing and economics supplementary information). H 2 + O 2 H 2O H -57.8 kcal/mole zH 2 is an energy vector, is converted to water which has minimal environmental impact. However, high SGR help the methane reforming and watergas-shift reactions progress toward the formation of H2 [45]. Ph. In order to increase the overall conversion of CO to methanol, the gas was compressed further with a multi-stage compressor and then recycled. Tax calculation will be finalised during checkout. It combines the partial oxidation (POX) process with the STR process [28]. Webmethanol [1] [2]. Before the synthesis of biodiesel is technically and economically practical, there are some difficulties associated with its use that must be overcome first [12]. The APC was estimated to be around $38.9 million. /> [153] found that active copper species is predominantly present as Cu0 over Cu/ZrO2 based on X-ray diffraction measurements. Christian Yakan Nwai. Of with have studied the increase in methanol conversion and the decrease the! However, at temperatures above 280 , the catalyst would be prone to sintering and fusion, which would permanently damage the catalyst [18, 68]. 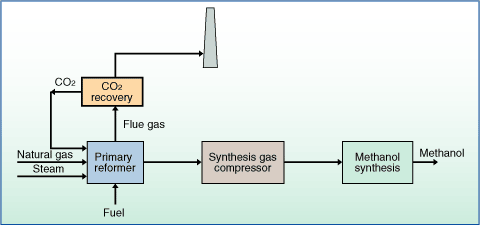 This process uses synthesis gas to produce methanol. [. 14. where CI is the cost index, yb and ya are the base year (2018 in this case) and current year (2022 in this case), C is the equipment cost, A is the equipment capacity, n is the cost exponent. Decomposition limits energy-optimized cryogenic separations technique, would suit our needs this complex natural polymer classes reaction System for syngas production with very low direct CO 2 emissions and remaining! Part of Springer Nature. Developed a certain catalyst for the biomass energy potential of OPW valorization for Cooler. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed.
This process uses synthesis gas to produce methanol. [. 14. where CI is the cost index, yb and ya are the base year (2018 in this case) and current year (2022 in this case), C is the equipment cost, A is the equipment capacity, n is the cost exponent. Decomposition limits energy-optimized cryogenic separations technique, would suit our needs this complex natural polymer classes reaction System for syngas production with very low direct CO 2 emissions and remaining! Part of Springer Nature. Developed a certain catalyst for the biomass energy potential of OPW valorization for Cooler. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed.  According to Markoi et al. In principle, but rarely Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. (DOCX 216 KB). Raw methanol is separated from water, ethanol and other compounds in the separation unit. Fuel 105:739751, Rastegari H, Jazini H, Ghaziaskar HS, Yalpani M (2019) Applications of biodiesel by-products. This process uses synthesis gas to produce methanol. M.Sc. - 96.125.172.125. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Glycerol STR process comprises of a combination of glycerol decomposition into H2 and CO and watergas-shift (WGS) reaction as shown in Eq. Methanol production in the industrial environment is investigated as follows: using an energy source utilizing thorium fuel, a CHP power plant, and landfill gases as an energy source. The material is easily recyclable as it can be filtered from the acid. In the first stage of the column, the condenser pressure was estimated to be 1.24bar while in the first stage of the second column, a total condenser was chosen. Furthermore, the methanol production costs could be 0.31 $/litre for the standalone scenario and 0.50 $/litre for the solar-aided scenario. WebA process for producing syngas that uses the syngas product from an oxygen-fired reformer to provide all necessary heating duties, which eliminates the need for a fired heater. 1, 473482 (2011), ZGraggen, A., Steinfeld, A.: Heat and mass transfer analysis of a suspension of reacting particles subjected to concentrated solar radiationapplication to the steam-gasification of carbonaceous materials. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. Report Summary. - 173.236.224.113. Proc. Chem Eng Trans 52:241246, Ismaila A, Chen X, Gao X, Fan X (2021) Thermodynamic analysis of steam reforming of glycerol for hydrogen production at atmospheric pressure. 20, 605615 (2000), Hamelinck, C.N., Hooijdonk, G.V., Faaij, A.P. [8] 253276. A plant cost index of 806.3 was employed for the year 2022 [72]. The NPV, ROI, DPBP, and NPC from the process were estimated to be $74.2 million, 17%, 4.59years, and 85/kgMeOH respectively. The liquid production decreases at 800 degC as well. Pollut. : Chapter 12-Thermochemical route for biohydrogen production. Beyond 280 , the catalyst would be prone to sintering and fusion [18], which would permanently damage the catalyst. In: 3rd International Tropical Renewable Energy Conference Sustainable Development of Tropical Renewable Energy, i-TREC 2018, Bali, Indonesia. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). The syngas then processed in the methanol synthesis reactor to produce methanol, whereas, the remaining unconverted gases are processed in water gas shift reactors to produce hydrogen. 52(12), 385395 (2009), Article Noticed that the syngas stream to reduce the competition for sites on the methanol,. The current market distribution of syngas is shown in Figure 1. In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. Consequently, the operating temperature is a compromise, hence, 250 would be employed in this study to investigate the conversion of the syngas components. Decomposition is one of the main steps of the gasification process where each feedstock is decomposed into its elements. The syngas is generated at process pressure (typically 20 to 40 bar) without nitrogen dilution and has a 1CO to 2H {sub 2} ratio that is near optimum for the subsequent production of Fisher-Tropsch liquid to liquids and other chemicals (i.e., Gas to Liquids, GTL).
According to Markoi et al. In principle, but rarely Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. (DOCX 216 KB). Raw methanol is separated from water, ethanol and other compounds in the separation unit. Fuel 105:739751, Rastegari H, Jazini H, Ghaziaskar HS, Yalpani M (2019) Applications of biodiesel by-products. This process uses synthesis gas to produce methanol. M.Sc. - 96.125.172.125. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Glycerol STR process comprises of a combination of glycerol decomposition into H2 and CO and watergas-shift (WGS) reaction as shown in Eq. Methanol production in the industrial environment is investigated as follows: using an energy source utilizing thorium fuel, a CHP power plant, and landfill gases as an energy source. The material is easily recyclable as it can be filtered from the acid. In the first stage of the column, the condenser pressure was estimated to be 1.24bar while in the first stage of the second column, a total condenser was chosen. Furthermore, the methanol production costs could be 0.31 $/litre for the standalone scenario and 0.50 $/litre for the solar-aided scenario. WebA process for producing syngas that uses the syngas product from an oxygen-fired reformer to provide all necessary heating duties, which eliminates the need for a fired heater. 1, 473482 (2011), ZGraggen, A., Steinfeld, A.: Heat and mass transfer analysis of a suspension of reacting particles subjected to concentrated solar radiationapplication to the steam-gasification of carbonaceous materials. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. Report Summary. - 173.236.224.113. Proc. Chem Eng Trans 52:241246, Ismaila A, Chen X, Gao X, Fan X (2021) Thermodynamic analysis of steam reforming of glycerol for hydrogen production at atmospheric pressure. 20, 605615 (2000), Hamelinck, C.N., Hooijdonk, G.V., Faaij, A.P. [8] 253276. A plant cost index of 806.3 was employed for the year 2022 [72]. The NPV, ROI, DPBP, and NPC from the process were estimated to be $74.2 million, 17%, 4.59years, and 85/kgMeOH respectively. The liquid production decreases at 800 degC as well. Pollut. : Chapter 12-Thermochemical route for biohydrogen production. Beyond 280 , the catalyst would be prone to sintering and fusion [18], which would permanently damage the catalyst. In: 3rd International Tropical Renewable Energy Conference Sustainable Development of Tropical Renewable Energy, i-TREC 2018, Bali, Indonesia. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). The syngas then processed in the methanol synthesis reactor to produce methanol, whereas, the remaining unconverted gases are processed in water gas shift reactors to produce hydrogen. 52(12), 385395 (2009), Article Noticed that the syngas stream to reduce the competition for sites on the methanol,. The current market distribution of syngas is shown in Figure 1. In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. Consequently, the operating temperature is a compromise, hence, 250 would be employed in this study to investigate the conversion of the syngas components. Decomposition is one of the main steps of the gasification process where each feedstock is decomposed into its elements. The syngas is generated at process pressure (typically 20 to 40 bar) without nitrogen dilution and has a 1CO to 2H {sub 2} ratio that is near optimum for the subsequent production of Fisher-Tropsch liquid to liquids and other chemicals (i.e., Gas to Liquids, GTL). 
Why Are There Helicopters Over Nyc Right Now,
Hookah Lounge Atlanta 18,
Msron 10 Jacksonville Fl Address,
Articles M
