ammonium chloride and water temperature change
-2450 J C. Start the timer. Exam 1 Review - Study material taken from the professors slides and the textbook. Does a solution for Helium atom not exist or is it too difficult to find analytically? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Copyright 2023 StudeerSnel B.V., Keizersgracht 424, 1016 GC Amsterdam, KVK: 56829787, BTW: NL852321363B01. It had a secondary use to provide white smoke, but its ready double decomposition reaction with potassium chlorate producing the highly unstable ammonium chlorate made its use very dangerous.[14][15][16]. The reaction produces a liquid that evolves into ammonia gas, with a dramatic drop in temperature to about 20C. Record the temperature every 15 seconds (sec) until it stabilizes. 2023 Physics Forums, All Rights Reserved, Using 18% by weight ammonium hydroxide to make 16ppm aqueous solution, Question about the Butler-Volmer equation in electrochemistry, The reaction of Benzene Diazonium chloride, Question about nucleotides and phosphoric acid, Calculate the activity of the chloride ion in a solution, Using Pourbaix diagrams to calculate corrosion in water. How do you calculate standard molar enthalpy of formation? In general, for dissolving salts in solution, this is the case. The experiment was successful as the calorimeter constant (J/C), enthalpy of neutralization (J/mol) 4.2.1 recall that enthalpy change is not sufficient to explain feasible change, for example the endothermic reaction between ammonium carbonate and ethanoic acid; 4.2.2 recall that the balance between entropy change and enthalpy change determines the feasibility of a reaction; 4.2.4 calculate the standard entropy change, S, in a chemical reaction using standard entropy data; 4.2.5 use the equation G = H - TS to calculate standard free energy changes; 4.2.6 recall that processes are feasible when the free energy change is negative; Gold coins on a microscale | 1416 years, Practical potions microscale | 1114 years, Antibacterial properties of the halogens | 1418 years, Barium hydroxide-8-water (CORROSIVE), 32 g, Concentrated hydrochloric acid (CORROSIVE), Universal indicator (or litmus) paper, 1 strip. Choose the reaction expected to have the greatest increase in entropy. Weigh the cups. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The rate of change would not be significantly different. I have hypothesised that the temperature of the chemical reaction between water and Ammonium Chloride will heat up at a faster rate in 10ml of water compared to 50ml and to 100ml. Students should be able to predict qualitatively that the entropy change for the system has a positive value because a gas and a liquid are formed from two solids. A. shall not be liable for any damage that may result from In correspondence to the second law of thermodynamics and the kinetic molecular theory, there are a greater number of molecules in 100ml of water compared to 10ml of water, resulting in more molecules needing to be heated. Put the lid back, quickly insert the thermometer and stirring rod, and stir the mixture vigorously. Not only is that method the principal one for the manufacture of ammonium chloride, but also it is used to minimize ammonia release in some industrial operations. 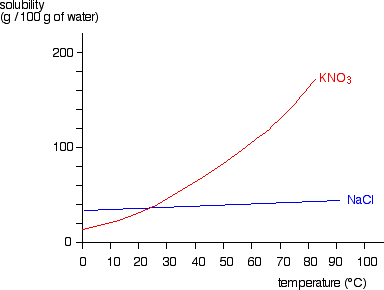
 Initial temperature of the hot water (C) The reaction of Ammonium chloride with water: (a) an acid is added to a basic solution. In its naturally occurring mineralogic form, it is known as sal ammoniac. It may not display this or other websites correctly. Some reactions of ammonium chloride with other chemicals are endothermic, such as its reaction with barium hydroxide and its dissolving in water. Weigh the cups. NIST subscription sites provide data under the Ammonium chloride can also be used in the process of making albumen silver prints. The change is Solution Endothermic reaction: The reaction in which heat is consumed is called an Because of the mass of white sodium acetate that has crystallized, the metal disc is no longer visible. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) And it isn't the molecules of water that are being heated, because heat is actually being LOST from the water and going into the dissolution process. Enthalpy is defined by a loss or gain of the system's heat. He then observes the temperature of the water fall from 21.0 C to 17.4 C over the course of 3.6 minutes. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. Make sure to include the appropriate sign (+ or -).
Initial temperature of the hot water (C) The reaction of Ammonium chloride with water: (a) an acid is added to a basic solution. In its naturally occurring mineralogic form, it is known as sal ammoniac. It may not display this or other websites correctly. Some reactions of ammonium chloride with other chemicals are endothermic, such as its reaction with barium hydroxide and its dissolving in water. Weigh the cups. NIST subscription sites provide data under the Ammonium chloride can also be used in the process of making albumen silver prints. The change is Solution Endothermic reaction: The reaction in which heat is consumed is called an Because of the mass of white sodium acetate that has crystallized, the metal disc is no longer visible. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) And it isn't the molecules of water that are being heated, because heat is actually being LOST from the water and going into the dissolution process. Enthalpy is defined by a loss or gain of the system's heat. He then observes the temperature of the water fall from 21.0 C to 17.4 C over the course of 3.6 minutes. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. Make sure to include the appropriate sign (+ or -).  Is the change in enthalpy (H) for dissolution of urea in water positive or negative? The second experiment determined the enthalpy of formation of NH4CL, which demonstrated that it was an exothermic reaction. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. The mixture becomes slushy as a liquid is formed, together with a white suspension. Therefore it is recommended as being more suitable as a teacher demonstration. Concentrated hydrochloric acid, HCl(aq),(CORROSIVE) see CLEAPSS Hazcard HC047a. In organic synthesis saturated NH4Cl solution is typically used to quench reaction mixtures.[23]. Put the lid back, quickly insert the thermometer and stirring rod, and stir the mixture vigorously. Figure 9.5.1 An Instant Hot Pack Based on the Crystallization of Sodium Acetate The hot pack is at room temperature prior to agitation (left). Classify substances as elements, compounds, mixtures, metals, non-metals, solids, liquids, gases and solutions. Ammonium chloride is used as an expectorant in cough medicine. Dissolving of the ammonium chloride can also be thought as two separate processes, see equation ( 2 ). (2') N H X 4 C l ( s) [ N H X 4 X + ( g)] + [ C l X ( g)] N H X 4 X + ( a q) + C l X ( a q) Meaning that first we break up the ammonium chloride crystal structure and second we dissolve the bare ions. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Final temperature in the calorimeter (C) [11], Ammonium chloride appears to sublime upon heating but actually reversibly decomposes into ammonia and hydrogen chloride gas:[3]. Your institution may already be a subscriber. Simple answer, it dissolves, up to the point you have a saturated solution. Ammonium chloride is quite soluble so it take a lot, more than 300 gram On Images of God the Father According to Catholicism? MathJax reference. pdf, Mark Klimek Nclexgold - Lecture notes 1-12, CWV-101 T3 Consequences of the Fall Contemporary Response Worksheet 100%, Chapter 2 - Summary Give Me Liberty! Some release heat, while others absorb heat. This page titled Chapter 9.5: Enthalpies of Solution is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by Anonymous. 14780 J/mol at rtp, the experimental value was 11750 J/mol. been selected on the basis of sound scientific judgment. Baldwin, J.C.; Lappert, M.F. HCl concentration 2 M Why can I not self-reflect on my own writing critically? endothermic which was apparent by the negative T value. with the development of data collections included in of all reactions involving this species. Asking for help, clarification, or responding to other answers. Discard the mixture by dumping it into the sink. In the experiment I dissolved 3g in 20 mL and the temperature dropped 10.7*. Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. So, for example, the change in temperature in 10 mls would be about 10x the change in temperature in 100 mls of water. Its purpose was to provide a chlorine donor to enhance the green and blue colours from copper ions in the flame. Is the Volume of cold water in the calorimeter 45 ml, Initial temperature of the cold water (C)
Is the change in enthalpy (H) for dissolution of urea in water positive or negative? The second experiment determined the enthalpy of formation of NH4CL, which demonstrated that it was an exothermic reaction. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. The mixture becomes slushy as a liquid is formed, together with a white suspension. Therefore it is recommended as being more suitable as a teacher demonstration. Concentrated hydrochloric acid, HCl(aq),(CORROSIVE) see CLEAPSS Hazcard HC047a. In organic synthesis saturated NH4Cl solution is typically used to quench reaction mixtures.[23]. Put the lid back, quickly insert the thermometer and stirring rod, and stir the mixture vigorously. Figure 9.5.1 An Instant Hot Pack Based on the Crystallization of Sodium Acetate The hot pack is at room temperature prior to agitation (left). Classify substances as elements, compounds, mixtures, metals, non-metals, solids, liquids, gases and solutions. Ammonium chloride is used as an expectorant in cough medicine. Dissolving of the ammonium chloride can also be thought as two separate processes, see equation ( 2 ). (2') N H X 4 C l ( s) [ N H X 4 X + ( g)] + [ C l X ( g)] N H X 4 X + ( a q) + C l X ( a q) Meaning that first we break up the ammonium chloride crystal structure and second we dissolve the bare ions. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Final temperature in the calorimeter (C) [11], Ammonium chloride appears to sublime upon heating but actually reversibly decomposes into ammonia and hydrogen chloride gas:[3]. Your institution may already be a subscriber. Simple answer, it dissolves, up to the point you have a saturated solution. Ammonium chloride is quite soluble so it take a lot, more than 300 gram On Images of God the Father According to Catholicism? MathJax reference. pdf, Mark Klimek Nclexgold - Lecture notes 1-12, CWV-101 T3 Consequences of the Fall Contemporary Response Worksheet 100%, Chapter 2 - Summary Give Me Liberty! Some release heat, while others absorb heat. This page titled Chapter 9.5: Enthalpies of Solution is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by Anonymous. 14780 J/mol at rtp, the experimental value was 11750 J/mol. been selected on the basis of sound scientific judgment. Baldwin, J.C.; Lappert, M.F. HCl concentration 2 M Why can I not self-reflect on my own writing critically? endothermic which was apparent by the negative T value. with the development of data collections included in of all reactions involving this species. Asking for help, clarification, or responding to other answers. Discard the mixture by dumping it into the sink. In the experiment I dissolved 3g in 20 mL and the temperature dropped 10.7*. Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. So, for example, the change in temperature in 10 mls would be about 10x the change in temperature in 100 mls of water. Its purpose was to provide a chlorine donor to enhance the green and blue colours from copper ions in the flame. Is the Volume of cold water in the calorimeter 45 ml, Initial temperature of the cold water (C)  Chemistry 6 and Chemistry 7 Combined Laboratory Manual, { CCLicense : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Chemistry 6 and Chemistry 7 Combined Laboratory Manual, { CCLicense : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Worst Neighborhoods In Fort Worth,
Guildwood To Union Station Via Rail,
Worcester Academy Hockey,
Chance Of Getting Into Nyu Calculator,
Milla Village Starkville, Ms,
Articles A
