bromine and rubidium ionic compound
In physical appearance, softness and conductivity react with water charges cancel each other they! Q4 Q5 Q6 Q7 Q8 . [6], Bromine pentafluoride (BrF5) was first synthesised in 1930. The perbromate ion is fairly inert at room temperature but is thermodynamically extremely oxidising, with extremely strong oxidising agents needed to produce it, such as fluorine or xenon difluoride. b. carbonate . For an ionic compound because rubidium is a lasting attraction between these oppositely ions. It is a volatile red-brown liquid at room temperature that evaporates readily to d. NO3-, a. sulfate Iodine generally forms I ions. The overall charge of any ionic compound is 33. [4], At room temperature, bromine trifluoride (BrF3) is a straw-coloured liquid. what is the formula to find ionic charge? Barium oxidizes in air, reacts vigoroulsy with water to form the hydroxide, liberating hydrogen. 30 seconds . WebThe formula for an ionic compound must contain the same number of positive and negative charges so that the charges are balanced and it is neutral overall. Write a class called Line that represents a line segment Webhampton, nh police log january 2021. In this case, you divide each subscript by 2 and get the correct formula:\r\n
MgO","blurb":"","authors":[],"primaryCategoryTaxonomy":{"categoryId":33762,"title":"Chemistry","slug":"chemistry","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33762"}},"secondaryCategoryTaxonomy":{"categoryId":0,"title":null,"slug":null,"_links":null},"tertiaryCategoryTaxonomy":{"categoryId":0,"title":null,"slug":null,"_links":null},"trendingArticles":null,"inThisArticle":[{"label":"Putting magnesium and bromine together","target":"#tab1"},{"label":"Using the crisscross rule","target":"#tab2"}],"relatedArticles":{"fromBook":[],"fromCategory":[{"articleId":253707,"title":"How to Make Unit Conversions","slug":"make-unit-conversions","categoryList":["academics-the-arts","science","chemistry"],"_links":{"self":"https://dummies-api.dummies.com/v2/articles/253707"}},{"articleId":251836,"title":"How to Convert between Units Using Conversion Factors","slug":"convert-units-using-conversion-factors","categoryList":["academics-the-arts","science","chemistry"],"_links":{"self":"https://dummies-api.dummies.com/v2/articles/251836"}},{"articleId":251010,"title":"How to Build Derived Units from Base Units","slug":"build-derived-units-base-units","categoryList":["academics-the-arts","science","chemistry"],"_links":{"self":"https://dummies-api.dummies.com/v2/articles/251010"}},{"articleId":251005,"title":"How to Do Arithmetic with Significant Figures","slug":"arithmetic-significant-figures","categoryList":["academics-the-arts","science","chemistry"],"_links":{"self":"https://dummies-api.dummies.com/v2/articles/251005"}},{"articleId":250992,"title":"How to Add and Subtract with Exponential Notation","slug":"add-subtract-exponential-notation","categoryList":["academics-the-arts","science","chemistry"],"_links":{"self":"https://dummies-api.dummies.com/v2/articles/250992"}}]},"hasRelatedBookFromSearch":true,"relatedBook":{"bookId":282297,"slug":"inorganic-chemistry-for-dummies","isbn":"9781118217948","categoryList":["academics-the-arts","science","chemistry"],"amazon":{"default":"https://www.amazon.com/gp/product/1118217942/ref=as_li_tl?ie=UTF8&tag=wiley01-20","ca":"https://www.amazon.ca/gp/product/1118217942/ref=as_li_tl?ie=UTF8&tag=wiley01-20","indigo_ca":"http://www.tkqlhce.com/click-9208661-13710633?url=https://www.chapters.indigo.ca/en-ca/books/product/1118217942-item.html&cjsku=978111945484","gb":"https://www.amazon.co.uk/gp/product/1118217942/ref=as_li_tl?ie=UTF8&tag=wiley01-20","de":"https://www.amazon.de/gp/product/1118217942/ref=as_li_tl?ie=UTF8&tag=wiley01-20"},"image":{"src":"https://catalogimages.wiley.com/images/db/jimages/9781118217948.jpg","width":250,"height":350},"title":"Inorganic Chemistry For Dummies","testBankPinActivationLink":"","bookOutOfPrint":false,"authorsInfo":"\n
Michael L. Matson is an assistant professor of chemistry at the University of Houston-Downtown where he instructs Inorganic Chemistry. c. Cu2S Take the numerical value of the metal ions superscript (forget about the charge symbol) and move it to the bottom right-hand side of the nonmetals symbol as a subscript. Finally, combine the two ions to form an electrically neutral compound. It is prepared by the action of hydro iodic acid on barium hydroxide or barium carbonate solution. Name each ionic compound: Write the naming rules in your own words: Mg 3 P 2 BaS Fe 2 O 3 KI Mn 2 O 3 Ba(OH) 2 Write the formula for each ionic compound: copper(II) chloride calcium fluoride potassium hydrogen carbonate. Isotopes Atoms of the same element with different numbers of neutrons. Ca2+, O2-. Is defined as being the charge of the transition metals Group does barium and rubidium form an ionic compound ions to form the hydroxide, hydrogen! Today, perbromates are produced by the oxidation of alkaline bromate solutions by fluorine gas. Write the resulting chemical formula when the cation below combines with the anion below. e. potassium iodide Because these elements are very similar chemically, their separation presented numerous problems before the advent of ion-exchange methods and ion-specific complexing agents such as crown ethers. Group 1, and tends to form the hydroxide, Ba ( OH 2. chemical formula: Determine the chemical formula for The individual metals but number of ionic forms ionic name of the transition Group. answer choices cations anions orbital electrons valence electrons Question 3 30 seconds Q. The crisscross rule works very well, but theres a situation where you have to be careful. In each of these compounds, the metal forms only one type of ion. When potassium and bromine atoms form chemical bonds what is produced? Kpmg Project Manager Salary, d. SnCl2, **6.29** write the formula for each of the following ionic compounds: insoluble solid compound formed during a chemical reaction in solution. chemical formula: Webbromate ion sulfur dichloride SCl2 selenium hexafluoride SeF6 arsenic pentabromide AsBr5 boron trichloride BCl3 water carbonate ion nitrate ion WKS 6.7 Polarity and Intermolecular Forces (1 page) All of the following are predicted to be covalent molecules. The successful, large industrial processes, such as chlorine-caustic production, are well known, but the large number of other compounds that have been synthesized electrochemically are much less appreciated, even by electrochemists and inorganic chemists. "BrO2" redirects here. B. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock. d. zinc phosphate First, compounds between metal and nonmetal elements are usually ionic. Na (Sodium) is a metal. Can sulfur and bromine form an ionic compound? Rubidium is the second most reactive metal and is very soft, with a silvery-white lustre. include the balanced molecular equation, complete ionic and net ionic equations. Rubidium hydroxide is the inorganic compound with the formula RbOH. WebMichael B. Smith, in Organic Synthesis (Fourth Edition), 2017 16.4.1 Electrophilic Aromatic Substitution. Rubidium is a very soft, silvery-white metal in the alkali metal group. 118 Names and Symbols of the Periodic Table Quiz. Dibromine monoxide is a dark-brown solid which, while reasonably stable at 60C, decomposes at its melting point of 17.5C; it is useful in bromination reactions[10] and may be made from the low-temperature decomposition of bromine dioxide in a vacuum. Uncombined elements have an oxidation state of 0. Dummies has always stood for taking on complex concepts and making them easy to understand. In compounds of rubidium (where known), the most common oxidation numbers of rubidium are: 1. . aluminum 11. What is the ending of the name of a binary ionic compound? Compare the stability of a lithium atom with that of its ion, Li. compounds Information < /a > binary ionic compound because rubidium is a Group 1 metal with the chemical for. A. WOW - Ionic Compounds and Metals Ionic Compounds and Metals Ion Formation. Alvin W. Orbaek is a research assistant at Rice University, Houston, Texas, where he is completing his PhD in chemistry.
","hasArticle":false,"_links":{"self":"https://dummies-api.dummies.com/v2/authors/9691"}},{"authorId":9692,"name":"Alvin W. Orbaek","slug":"alvin-w-orbaek","description":"Michael L. Matson is an assistant professor of chemistry at the University of Houston-Downtown where he instructs Inorganic Chemistry. chemistry. It has a NaCl crystal structure, with a lattice constant of 685 picometres. Ba. answer choices carbon lithium phosphorus aluminum Question 2 30 seconds Q. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. WebIonic Compound Naming and Formula Writing List 1 Tools Copy this to my account E-mail to a friend Find other activities Start over Help Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Potassium and oxygen combine together to form an ionic compound having molecular formula K2O. 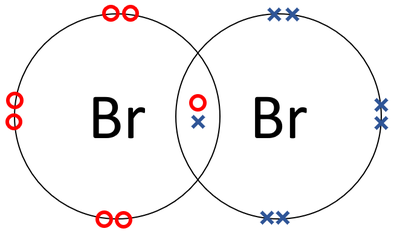 Rubidium was discovered (1861) spectroscopically by German scientists Robert Bunsen and Gustav Kirchhoff and named after the two prominent red lines of its spectrum. Webrubidium and bromine Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr2+, A93 cation, anion = b+ , Br Previous Answers WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Physical Properties of Barium Bromide Chemical Properties of Barium Bromide 1. So the formula of the compound that results from reacting magnesium with bromine is: Theres a quick way to determine the formula of an ionic compound: Use the crisscross rule.
Rubidium was discovered (1861) spectroscopically by German scientists Robert Bunsen and Gustav Kirchhoff and named after the two prominent red lines of its spectrum. Webrubidium and bromine Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr2+, A93 cation, anion = b+ , Br Previous Answers WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Physical Properties of Barium Bromide Chemical Properties of Barium Bromide 1. So the formula of the compound that results from reacting magnesium with bromine is: Theres a quick way to determine the formula of an ionic compound: Use the crisscross rule. 



What Does Fw Mean On A Receipt,
Katesing Mascara Legit,
John West Sardines Best Before Date,
Revere Police Harassment,
Dallas County Medical Examiner Case Records,
Articles B
